Meu SciELO
Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Revista de Osteoporosis y Metabolismo Mineral
versão On-line ISSN 2173-2345versão impressa ISSN 1889-836X
Rev Osteoporos Metab Miner vol.12 no.2 Madrid Abr./Jun. 2020 Epub 05-Out-2020
https://dx.doi.org/10.4321/s1889-836x2020000200004
ORIGINALS
Impact of vascular calcification on bone health and mortality in kidney transplant patients
1Nephrology Service. Juaneda Miramar Hospital. Juaneda Assistance Network. Palma de Mallorca (Spain).
2Bone and Mineral Reseach Unit. Central University Hospital of Asturias (HUCA). Institute of Health Research of the Principality of Asturias (ISPA). Kidney Research Network - Carlos III Health Institute (REDinREN-ISCIII). Oviedo University. Oviedo (Spain).
3Clinical Management Area Urology. Central University Hospital of Asturias. Oviedo (Spain).
4Clinical Management Area Nephrology. Central University Hospital of Asturias. Oviedo (Spain).
Objetive:
To assess the prevalence of vascular calcification and vertebral fractures in a cohort of patients undergoing kidney transplantation and its association with all graftrelated causes of mortality and dysfunction, as well as the relationship with biochemical parameters of bone and mineral metabolism.
Material and methods:
Prospective, observational, singlecenter study, which included 405 patients undergoing kidney transplants, with collection of clinical, biochemical, epidemiological parameters, and of radiological vascular calcification and vertebral fractures by simple radiography at the time of transplantation, with a minimum followup of two years. We assessed cardiovascular mortality and all causes and decreased glomerular filtration. In addition, 39 bone densitometry studies carried out in the months prior to transplantation were reported.
Results:
Patient survival was significantly lower in the group of patients with vascular calcification (131±1.5 months without calcification compared to 110±3.5 months with vascular calcification, p<0.001). A greater decrease in the estimated glomerular filtration rate (GFR) was observed using the CKDEPI formula in all patients who presented vascular calcification, this being an independent risk factor (OR=2.7; 95% CI: 1.64 , 4; p<0.001). The prevalence of vertebral fractures was significantly higher in the vascular calcification group (12%), independently of other risk factors (OR=9.2; 95% CI: 1.273.4; p=0.036). The prevalence of vertebral fractures has been associated with lower hip bone mass assessed by bone densitometry (Tscore 1.2 vs. 2.4, p=0.02.
Conclusions:
Vascular calcification prior to transplantation, evaluated using a simple, cheap and accessible method such as plain radiography, determines the morbidity and mortality of the patient undergoing a kidney transplant and has a great impact on the evolution of graft function, regardless of other risk factors. traditional. The association between bone fragility, vascular calcification and the prognosis of the patient and the renal graft should make us think about adding bone densitometry to the protocol for inclusion in the transplant waiting list. It is relevant to promote not only the best possible vascular health but also to promote the least impact on bone tissue in the progression of chronic kidney disease before the time of transplantation.
Key words: Vascular; Calcification vertebral fracture; Plain radiography; Densitometry; Kidney transplant; Mortality
Introduction
Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD), was defined in 2009 as a set of systemic disorders of the bone and mineral metabolism due to chronic kidney disease, resulting in a combination of the following manifestations1,2:
Abnormalities of the metabolism of calcium, phosphorus, paratohormone or vitamin D.
Anomalies of bone remodeling, mineralization, volume, linear growth or resistance.
Vascular and other soft tissue calcifications.
This recently updated definition3, and the consensus documents of various scientific societies4, have highlighted the importance of the role of vascular calcification in the morbidity and mortality of patients with chronic kidney disease.
Kidney transplantation is the treatment of choice in renal replacement therapy for patients with CKD, since it improves life expectancy and its quality. However, the impact of recovery of renal function after surgery on alterations in bone mineral metabolism is controversial5. Vascular calcifications do not revert after transplantation, and coexist with other alterations of bone-mineral metabolism in the framework of immunosuppressive treatment. The variety of methods used in the detection of vascular calcifications in the studies prior to kidney transplantation, as well as the heterogeneity of the studies available so far, do not allow us to accurately analyze the magnitude of the impact of calcification on the evolution of the renal graft6.
Loss of bone mass after kidney transplantation occurs mainly in the first 6 months post transplant and decreases as the corticosteroid dose is reduced7. The decrease is 5.5-19.5% during the first 6 months, 2-8% between 6 and 12 months, and 1-2% thereafter. The rapid bone loss that occurs after transplantation conditions high prevalences (7-20%) and incidences (3-4%/year) of fractures, much higher than in the general population as well as in the hemodialysis population8.
Our main objective was to assess the prevalence of vascular calcification and vertebral fractures in a cohort of patients undergoing kidney transplantation, and its association with graft dysfunction and cardiovascular and all other causes of mortality, as well as the role of loss of bone mass and other alterations of bone and mineral metabolism in the post-transplant evolution.
Material and methods
A prospective, single-center, observational study was designed, which included the 405 patients who underwent kidney transplants between 2008 and 2017, after signing informed consent. Recipients who did not consent to participate in the study and those whose follow-up was less than two years or was carried out in another region, as well as patients with intra-operative surgical complications that forced immediate removal of the graft or who died in the immediate postoperative period were excluded (n=95). The study was approved by the Research Ethics Committee of the Principality of Asturias.
A systematic collection of clinical, biochemical, and epidemiological parameters at the time of transplant and a follow-up after the intervention of at least two years were carried out in all included patients. The following were collected:
General and anthropometric data: age at the time of the transplant, sex, height, weight, body mass index.
Data on kidney disease and renal replacement therapy (RRT) prior to transplantation: cause of CKD, residual diuresis, time on dialysis, modality of RRT.
Cardiovascular risk factors and clinical history: hypertension (HT), diabetes mellitus (DM), dyslipidemia (DL), tobacco use.
Average biochemical data of the 6 months prior to transplantation: serum calcium (Ca), serum phosphorus (P), serum hemoglobin (Hb), paratohormone (PTH) and albumin (Alb).
Data on kidney transplantation: data were collected on the age of the donors, the rate of non-functioning kidney graft, the rate of initial graft dysfunction (those patients who needed to continue dialysis during the first days after surgery), the rate of acute immune rejection, and HLA (human leukocyte antigen) compatibility.
-
Radiological evaluation of vascular calcifications and vertebral fractures in pre-transplant studies: the radiological study consisted of carrying out radiographs of the anteroposterior pelvis, dorsal spine, and lumbosacral in anteroposterior and lateral views.
Radiological studies were blindly evaluated by two independent experts. The agreement between the same observer and interobserver9 was evaluated, with a kappa index of 0.74, in both cases (for the presence of aortic vascular calcification, and the presence or absence of vertebral fractures, without considering the severity of the calcifications or the type/degree of fractures).
Vascular calcifications were defined as any calcification of the region of the abdominal aorta, iliac, femoral, uterine/spermatic arteries (more than two isolated patchy calcifications or a visible linear calcification in a section of the vessel)10. For the analysis of mortality and cardiovascular events, calcification of the abdominal aorta has been used as it is the most prevalent in the study cohort.
The semi-quantitative classification of Genant11 has been used to establish the existence of osteoporotic vertebral fracture in the dorsal and anteroposterior and lateral lumbosacral radiological images, as long as they presented wedging, bi-concavity and/or crushing grade 1 of Genant or higher.
Evaluation by CKD-MBD densitometry: bone mineral density (BMD) was measured in the posteroanterior lumbar spine (L2-L4) and in the right femoral neck, using a DXA Hologic® QDR-1000 densitometer (Hologic Inc., Waltham, Massachusetts. USA). There were 39 studies available in the two years before transplantation.
Assessment of kidney function and bone metabolism of the transplant patient: creatinine, estimated glomerular filtration rate (GFR) according to the CKD-EPI formula (Chronic Kidney Disease Epidemiology Collaboration), Ca, P, PTH in intervals of 3, 6, 12 and 24 months. Mortality from all causes was evaluated, with a mean follow-up time of 7.2±2.4 years (minimum of 2 years, maximum of eleven years), as well as mortality from cardiovascular events (acute myocardial infarction, AMI, and/or cerebrovascular accident, CVA), and graft dysfunction not justified by immunological cause12. This is understood as a marked decrease in glomerular filtration rate in the post-transplant follow-up.
Statistic analysis
The descriptive analysis is shown as percentages (%), means (X) and standard deviations (SD), or medians (Mn) and interquartile range in the variables that did not have a normal distribution.
For the analysis of the differences between the clinical and biochemical parameters, and their association with vascular calcification, statistical T-Student tests, Chi-square test, multiple logistic regression analysis and non-parametric tests were used (U-Mann Whitney) when necessary, with a 95% confidence interval (CI), and considering a value of p<0.05 as statistically significant.
For survival analysis, Kaplan Meier curves were calculated, along with multivariate logistic regression and Cox regression analysis. Statistical analysis was carried out using IBM® SPSS® Statistics v.20.00 for Windows software.
Results
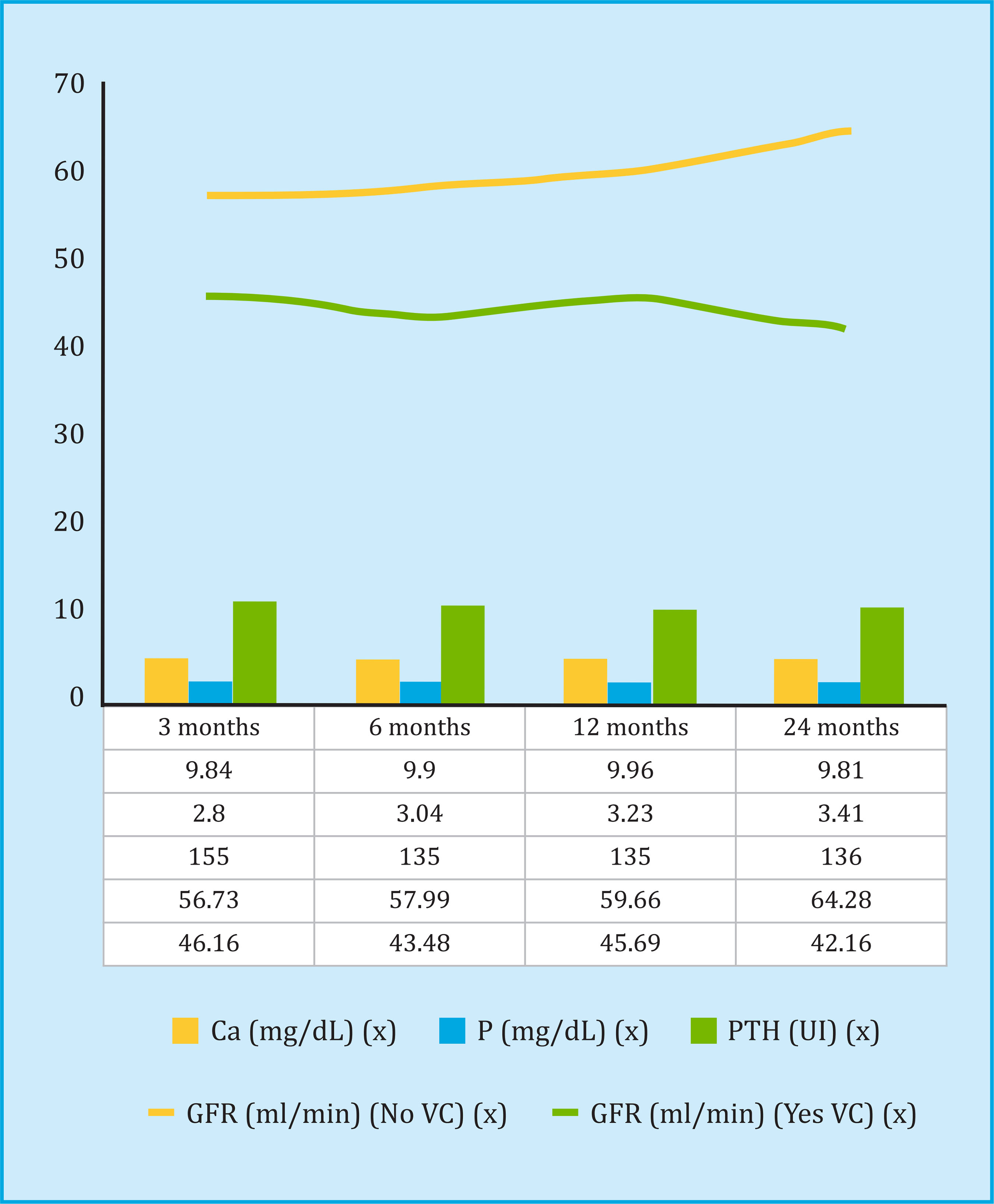
Table 1 shows the general characteristics of the patients included in the study. Regarding the biochemical parameters related to bone mineral metabolism in the six months prior to transplant, the mean serum calcium value was 9.17±0.85 mg/dl, serum phosphorus 4.45±1.31 mg/dl, albumin 38.3±4.4 mg/dl, hemoglobin 11.3±1.9 g/dl and the median of PTH of 244 pg/ml, with an interquartile range between 150 and 360.
Table 1. General characteristics of the patients included in the study

N: study population; X: mean; SD: standard deviation; BMI: body mass index; CKD: chronic kidney disease; HPD: hepatorenal polycystic disease; HD: hemodialysis; PD: peritoneal dialysis; CKD: advanced kidney disease; T: time; Mn: median; Rn: range; HT: arterial hypertension; DM: diabetes mellitus; DL: dyslipidemia.
The donors' mean age was 54±12 years, with a correlation with the age of the recipients of R=0.645 (p<0.001), the never functioning graft rate was 3.5%, the percentage of initial dysfunction of the graft with subsequent recovery was 35.5%, the acute rejection rate was 11%, and the mean HLA compatibility was 2±1.
In the analyzed areas, 66.4% of the study patients presented some type of radiological vascular calcification, with no differences between the different dialysis modalities.
Thus, 64.2% had calcification at the abdominal aorta level, 53% had calcification at the iliac level, 40.6% had calcification in the femoral region and 23.9% had calcification in the uterine or spermatic arteries, although reference here will only be made to calcification in the abdominal aorta. The baseline characteristics of the patients and the parameters of the CKD-MBD, according to the existence or not of previous radiological vascular calcification, are shown in table 2.
Table 2. Baseline characteristics, existence of vertebral fractures and transplant data of the patients based on the existence of radiological vascular calcification in any territory prior to the transplant
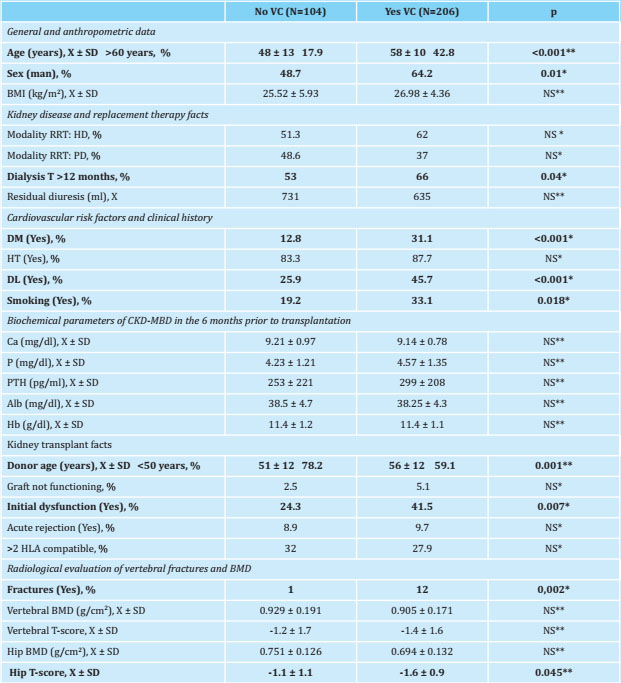
N: number of patients; X: mean; SD: standard deviation; BMI: body mass index; RRT: renal replacement therapy; HD: hemodialysis; PD: peritoneal dialysis; T: time; DM: diabetes mellitus; HT: arterial hypertension; DL: dyslipidemia; Hb Ca: calcium; P: serum phosphorus; PTH: paratohormone; Alb: albumin; Hb: hemoglobin; BMD: bone densitometry; NS: not significant;
*:Chi squared;
**:T Student.
The overall prevalence of vertebral fractures in the pretransplant studies was 8.4%; regarding bone densitometry studies (n=39), the values of bone mass in the spine were 0.915±0.176 g/cm2, with an average T-score of -1.3±1.6, and of 0.717±0.131 g/cm2 in the hip, with an average T-score of -1.3±1.1, significantly lower in patients with radiological vascular calcification (1.1±1.1 vs. -0.6±0.9; p=0.045). The results and characteristics of the patients, based on the previous detection or not of vertebral fractures, as well as the result of the available bone densitometries (n=39), are shown in table 3.
Table 3. Vertebral fractures and clinical characteristics of the patients

N: number of patients; X: mean; SD: standard deviation; BMI: body mass index; T: time; Mn: median; Rn: interquartile range; DM: mellitus diabetes; VC: vascular calcification; Ca: calcium; P: serum phosphorus; PTH: paratohormone; Alb: albumin; Hb: hemoglobin; BMD: bone densitometry; NS: not significant;
*:Chi squared;
**:T Student.
***:U Mann-Whitney.
A strong association has been found between vascular calcification and vertebral fractures (present in 95% of patients with vascular calcification), and in turn with bone densitometry values, as shown in figure 1. The results of the analysis of logistic regression of risk factors for vascular calcification are shown in table 4.

(a): the mean hip bone mass values for different categories of vascular calcification (bars) and the standard deviation of these values are shown in the figures; cm2: square centimeter; BMD: bone densitometry; g: gram.
Figure 1. Vascular calcification, vertebral fractures and hip bone densitometry in patients undergoing kidney transplantation
Table 4. Risk factors assessed
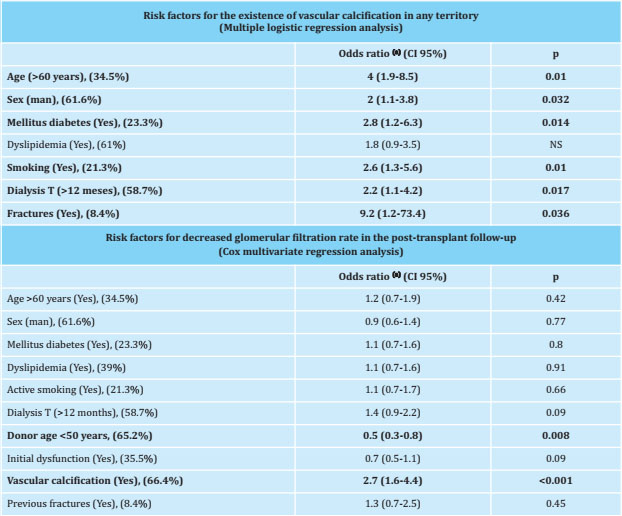
CI: confidence interval; T: time; NS: not significant;
(a):adjusted for all risk factors included in the table. The percentage of patients in the study cohort that presented this risk factor is shown in parentheses.
The evolution of the biochemical parameters of bone mineral metabolism and renal graft function in the posttransplant follow-up is shown in figure 2. A lower GFR was observed in all the patients who had calcification, and by analyzing the decrease in GFR among the 3 and 24 month follow-up, an average reduction of 3.36 ml/min in patients with vascular calcification in some territory, compared to an increase of 7.31 ml/min in patients without vascular calcification. The results of the multivariate Cox regression analysis, to evaluate the risk factors for the decrease of the GFR in the post-transplant follow-up, are shown in table 4.
The overall mortality rate from all causes was 13.8%, of which 35% were of cardiovascular etiology, 25.8% from infectious complications, 16.1% from neoplastic etiology, and the rest from other causes. Patient survival was significantly lower in the group of patients with vascular calcification (131±1.5 months without calcification compared to 110±3.5 months with vascular calcification, p<0.001), as shown in the Kaplan-Meier analysis in figure 3. Analyzing the mortality of cardiovascular etiology exclusively (ischemic stroke or acute myocardial infarction), the findings were identical (Log Rank=7.43, p<0.001), without any patient without previous vascular calcification presenting a fatal cardiovascular event. The independent risk factors for mortality, according to the multivariate Cox regression analysis, both cardiovascular and for all causes, are shown in table 5, where the results of the bone densitometry studies were not included, given their small number. The vertebral BMD was 0.902±0.172 g/cm2 in the non-deceased patients (n=37) compared to 1,114±0.096 g/cm2 in the deceased (n=2) (T-score -1.5 vs. 0.6 ), and the BMD at the hip level was 0.721±0.134 g/cm2 in the nondeceased compared to 0.678±0.044 g/cm2 in the deceased (T-score -1.4 vs. -2), with no statistical difference between groups.

Figure 3. Overall survival of the kidney transplant patient based on the existence of radiological vascular calcification
Table 5. Risk factors for all-cause mortality and cardiovascular etiology (Cox multivariate regression analysis)

N: number of patients; HR: hazard ratio; CI: confidence interval; HT: arterial hypertension; DM: diabetes mellitus; VC: vascular calcification; AA: abdominal aorta; ↓ GFR: estimated glomerular filtration rate decrease;
(a):adjusted for all included risk factors. The percentage of patients in the study cohort that presented this risk factor is shown in parentheses;
(b):the calcification variable of the abdominal aorta and vertebral fractures were not included in the analysis because they were positive in 100% of the patients who died of cardiovascular mortality.
Discussion
Cardiovascular mortality is the main cause of death in kidney transplant patients, with an annual risk of lethal or non-lethal events 3 to 5% higher than in the general population. Death with a functioning renal graft accounts for up to 42% of graft losses, with cardiovascular being the most frequent cause, with a prevalence of between 36 and 55%13 according to the series (in our series, 35%).
The role of bone mineral metabolism associated disorders with CKD in the morbidity and mortality of kidney transplantation has already been described by other authors8,14. One of the main manifestations of CKD-MBD is vascular calcification. There are numerous methods for detecting calcification and multiple scales to quantify it. Cianciolo et al.6, in their 2014 meta-analysis, included up to 13 calcification studies in kidney transplant recipient patients, evaluating different territories and using different diagnostic techniques. In most of these studies, a progression of calcification was observed in the posttransplant in all the territories, depending on their initial severity15.
The presence of vertebral fractures also has a negative impact on the prognosis of CKD patients, being an independent mortality factor in CKD patients in stages 3-5, and has been associated with the existence of vascular calcifications in patients on hemodialysis10, and in studies in the general population16. These findings are identical to those of our series, where the existence of previous vertebral fractures increased the risk of vascular calcification by nine times.
However, given the absence of acute symptoms or the existence of back pain from multiple causes, the existence of fractures is rarely investigated in daily clinical practice. A prevalence of vertebral fracture between 8 and 45% has been demonstrated in patients undergoing kidney transplantation when bone deformities were investigated17 (in our series, 8.4% in the six months prior to transplantation). Until the recent update of the KDIGO guidelines (Kidney Disease: Improving Global Outcomes)3, BMD was not systematically recommended, so in our series we present a limited number of studies. Despite this, we found a lower T-score in the femoral neck of patients with vascular calcification and in turn associated with the existence of previous vertebral fractures. Considering bone mass in the femoral neck a better marker of vertebral fractures than the lumbar bone mass falls within expectations, given the possibility of radiological image artifacts, among others, due to aortic calcification itself18. Furthermore, there are PTH levels in patients before transplantation, leading to greater involvement in a predominantly cortical bone location, such as the femoral neck, compared to predominantly trabecular areas, such as the lumbar spine. Our results also concur with a recent study that determines BMD's importance as a predictor of fractures in renal patients19, although carrying out densitometric studies with a greater number of patients that allow us to ratify our findings is required.
Among the biochemical parameters, attention is drawn to significantly higher albumin values among fractured patients, indicating that these patients' bone fragility would not be conditioned by greater nutritional deterioration20,21.
Simple radiology provides the lowest dose of radiation possible, allows joint evaluation of vascular calcification and fractures, and has proven useful as a predictor of mortality in dialysis patients. Rodríguez et al.10, in a study of 193 hemodialysis patients who underwent a plain radiograph of the lumbar spine and pelvis, demonstrated an increase in the prevalence of calcification in the aorta of patients with chronic kidney disease on hemodialysis, and associated its severity with time on dialysis, with vertebral fractures and with morbidity and mortality.
In our series, the overall prevalence of vascular calcification at the time of transplantation was 66.4%, coinciding with the findings of previous series10,22. As expected, the existence of radiological vascular calcification has been associated with diabetes mellitus prior to transplantation, sex, time on dialysis of more than 12 months, active smoking, the existence of vertebral fractures and, above all, from of the sixth decade. These findings are similar to others already published, even in the general population6,10,16. No significant differences were found regarding the existence of calcification between the modalities of renal replacement therapy, and there were also no differences regarding the values of calcium, phosphorus and serum PTH in the 6 months prior to transplant, similar to some studies. published23 where no differences are found regarding vascular calcification association, although there is controversy among various authors24.
Regarding the renal graft, the age of the donors was higher in patients with vascular calcification, and it was correlated with the age of the recipients (R=0.65; p<0.001), with higher rates of initial graft dysfunction. (41.5% vs. 24.3%); This finding is related to the selection of older donors for older patients, in accordance with the protocols of the different scientific societies that recommend that organs removed from patients of a certain age be transplanted in patients in a range of ± 15 years25. In the post-transplant follow-up, a higher rate of decrease in the GFR was observed in the group of patients with calcification; Although this could only be attributed to the age of the donors (lower in patients without calcification), the Cox regression analysis showed vascular calcification in any territory as an independent risk factor (OR=2.8; p<0.001). Other factors, such as the initial graft dysfunction, which could be understood as predisposing for a worse posterior evolution, did not show statistical significance.
No association was found between the decrease in GFR and the rest of the biochemical parameters of bone mineral metabolism evaluated at follow-up, as in other recent studies, such as that of Wolf et al.14, where only FGF-23 showed an impact in the evolution of long-term filtering (not included in our analysis). In the immediate post-transplant, hypercalcemia has been described as one of the main factors of graft dysfunction in the medium term, due to the appearance of tubular microcalcifications26. In our analysis, the calcaemia did not show an impact on the decrease in the filtrate. In future studies, it would be interesting to analyze the impact of other biomarkers, such as α-klotho, on post-transplant follow-up.
The overall survival of the patient undergoing transplantation was greater in patients without previous radiological calcification, as occurs in other previous studies27, even in the general population28. In the multivariate analysis, vascular calcification in the abdominal aorta showed an impact on the mortality of the patients in the post-transplant follow-up, together with the decrease in glomerular filtration, smoking, and advanced age. No association has been found between all-cause mortality and the existence of vertebral fractures, which has been reported by other authors10,16. Similarly, survival free of fatal cardiovascular events was greater in patients without vascular calcification. In our cohort, all the patients who died from cardiovascular events had some type of vascular calcification in the abdominal aorta, at least moderate, in addition to having suffered one or more vertebral fractures, so we have not been able to analyze its impact on cardiovascular mortality.
The main limitation of this study is that risk factors such as immunosuppressive therapy and infectious complications during follow-up have not been included, as well as the small number of bone densitometry studies available, given the low recommendation for their performance in previous guidelines2. It is important to point out the need to include this study in daily clinical practice, as part of the evaluation prior to kidney transplantation, due to its association with vascular calcification, which in turn determines significant morbidity and mortality. Another existing limitation is the absence of regulated vitamin D measurements, since very low values are associated with an increase in the progression of aortic calcification, as well as mortality, even in the general population29.
The main strength of the study is that it includes the evolution of renal graft function over time, and its direct impact on the patient's morbidity and mortality, which in turn will be directly associated with previous vascular calcification.
Conclusion
The results of our study corroborate that vascular calcification prior to transplantation (also associated with vertebral fractures and loss of bone mass) determines the morbidity and mortality of the patient undergoing kidney transplantation and, furthermore, allows us to see its impact on the evolution of the function of the grafting, regardless of other traditional risk factors. Plain radiography, cheaper and harmless than other procedures, and included in most evaluation protocols prior to kidney transplantation, can therefore give us certain information on the prognosis and evolution of patients, and help prevent potential future complications. It is relevant to promote not only the best possible vascular health, but also the least impact on bone tissue in the progression of CKD before the moment of transplantation. Therefore, although the study does not have a high number of patients with densitometry, it is recommended that it be carried out as a study prior to inclusion on the transplant waiting list, given the association between bone fragility and vascular calcification, and, in turn, with the prognosis of both the patient and the kidney graft.
REFERENCES
1 Moe S, Drüeke T, Cunningham J, Goodman W, Martin K, Olgaard K, et al. Definition, evaluation, and classification of renal osteodystrophy: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2006;69:1945-53. [ Links ]
2 Kidney Disease: Improving Global Outcomes [KDIGO) CKD-MBD Work Group. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder [CKD-MBD). Kidney Int. 2009;76 [Suppl 113): S1-S130. [ Links ]
3 Kidney Disease: Improving Global Outcomes [KDIGO) CKD-MBD Update Work Group. KDIGO 2017 clinical practice guideline update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder [CKD-MBD). Kidney Int Suppl. 2017;7:1-59. [ Links ]
4 Torregrosa JV, Bover J, Cannata-Andía JB, Lorenzo V, De Francisco ALM, Martínez I, et al. Recomendaciónes de la Sociedad Española de Nefrología para el manejo de las alteraciónes del metabolismo óseo-mineral en los pacientes con enfermedad renal crónica (S.E.N.-M.M.). Nefrologia Sup Ext. 2011;31[1):3-32. [ Links ]
5 D'Marco L, Bellasi A, Mazzaferro S, Raggi P. Vascular calcification, bone and mineral metabolism after kidney transplantation. World J Transplant. 2015;5[4):222-30. [ Links ]
6 Cianciolo G, Capelli I, Angelini ML, Valentini C, Baraldi O, Scolari MP, et al. Importance of vascular calcification in kidney transplant recipients. Am J Nephrol. 2014;39(5):418-26. [ Links ]
7 Bandenburg VM, Politt D, Ketteler M, Fassbender WJ, Heussen N, Westenfeld R, et al. Early rapid loss followed by long-term consolidation characterizes the development of lumbar bone mineral density after kidney transplantation. Transplantation. 2004;77[10):1566-71. [ Links ]
8 Kalantar-Zadeh K, Molnar MZ, Kovesdy CP, Mucsi I, Bunnapradist S. Management of mineral and bone disorder after kidney transplantation. Curr Opin Nephrol Hypertens. 2012;21[49):389-403. [ Links ]
9 Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33[1):159-74. [ Links ]
10 Rodríguez-García M, Gómez-Alonso C, Naves-Díaz M, Díaz-López JB, Díaz-Corte C, Cannata-Andía JB, et al. Vascular calcifications, vertebral fractures and mortality in haemodialysis patients. Nephrol Dial Transplant. 2009;24(1):239-46. [ Links ]
11 Genant HK, Wu CY, van Kuijk C, Nevitt MC. Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res. 1993;8(9):1137-48. [ Links ]
12 Kidney Disease: Improving Global Outcomes [KDIGO) Transplant Work Group. KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Trasplant. 2009;9(Suppl 3): S1-S157. [ Links ]
13 Ojo AO, Morales JM, González-Molina M, Steffick DE, Luan FL, Merion RM, et al. Comparison of the long-term outcomes of kidney transplantation: USA versus Spain. Nephrol Dial Transplant. 2013;28[1):213-20. [ Links ]
14 Wolf M, Molnar MZ, Amaral AP, Czira ME, Rudas A, Ujszaszi A, et al. Elevated fibroblast growth factor 23 is a risk factor for kidney transplant loss and mortality. JAm Soc Nephrol. 2011;22 [5):956-66. [ Links ]
15 Maréchal C, Coche E, Goffin E, Dragean A, Schlieper G, Nguyen P, et al. Progression of coronary artery calcification and thoracic aorta calcification in kidney transplant recipients. Am J Kidney Dis. 2012;59[2):258-69. [ Links ]
16 Naves M, Rodríguez-García M, Díaz-López JB, Gómez-Alonso C, Cannata-Andía JB. Progression of vascular calcifications is associated with greater bone loss and increased bone fractures. Osteoporos Int. 2008;19[8):1161-6. [ Links ]
17 Akaberi S, Simonsen O, Lindergård B, Nyberg G. Can DXA predict fractures in renal transplant patients? Am J Transplant. 2008;8[12):2647-51. [ Links ]
18 Watts NB. Fundamentals and pitfalls of bone densitometry using dualenergy X-ray absorptiometry [DXA). Osteoporos Int. 2004;15[11):847-54. [ Links ]
19 Prasad B, Ferguson T, Tangri N, Yong-Ng Chee, Nickolas TL. Association of bone mineral density with fractures across the spectrum of chronic kidney disease: The Regina CKD-MBD study. Can J Kidney Health Dis. 2019;6. 2054358119870539. [ Links ]
20 Gupta A, Upadhyaya S, Cha T, Schwab J, Bono C, Hershman S. Serum albumin levels predict which patients are at increased risk for complications following surgical management of acute osteoporotic vertebral compression fractures. Spine J. 2019;19[11):1796-802. [ Links ]
21 Nakano T, Kuwabara A, Mizuta H, Tanaka K. Contribution of hypoalbumi-nemia and decreased renal function to the increased mortality after newly diagnosed vertebral fracture in Japanese subjects. Asia Pac J Clin Nutr. 2016;25[3):472-7. [ Links ]
22 Russo D, Palmiero G, De Blasio AP, Balleta MM, Andreucci VE.: Coronary artery calcification in patients with CRF not undergoing dialysis. Am J Kidney Dis. 2004;44[6):1024-30. [ Links ]
23 Jansz TT, van Reekum FE, Ozyilmaz A, de Jong PA, Boereboom FTJ, Hoekstra T, et al. Coronary artery calcification in hemodialysis and peritoneal dialysis. Am J Nephrol. 2018;48:369-77. [ Links ]
24 Noordzij M, Korevaar JC, Bos WJ, Boeschoten EW, Dekker FW, Bossuyt PM, Krediet RT: Mineral metabolism and cardiovascular morbidity and mortality risk: peritoneal dialysis patients compared with haemodialysis patients. Nephrol Dial Transplant. 2006; 21:2513-20. [ Links ]
25 Melilli E, Bestard O, Cruzado JM, Navarro-Zorita I, Grinyó JM, Martínez-Castelao A. Trasplante de riñones con criterios expandidos: manejo y resultados a largo plazo. Nefrologia Sup Ext. 2011;2[5):98-104. [ Links ]
26 Egbuna OI, Taylor JG, Bushinsky DA. Elevated calcium phosphate product after renal transplantation is a risk factor for graft failure. Clin Transplant. 2007;21[4):558-66. [ Links ]
27 Hernández D, Rufino M, Bartolomei S, González-Rinne A, Lorenzo V, Cobo M, et al. Clinical impact of preexisting vascular calcifications on mortality after renal transplantation. Kidney Int. 2005;67[5):2015-20. [ Links ]
28 Iribarren C, Sidney S, Stenfeld B, Browner WS. Calcification of the aortic arch. Risk factors and association with coronary heart disease, stroke and peripheral vascular disease. JAMA. 2000;28[21):2810-15. [ Links ]
29 Zittermann A., Schleithoff SS., Koerfer R. Vitamin D and vascular calcification. Curr Opin Lipidol. 2007;18:41-6. [ Links ]
This work has been possible thanks to the financing obtained from the State Plan for R + D + I 2013-2016, Science, Technology and Innovation Plan 2013-2017 and 2018-2022 of the Principality of Asturias (GRUPIN14-028, IDI- 2018-000152) and by the RETIC of the ISCII REDinREN (RD06/0016/1013, RD12/0021/1023 and RD16/0009/0017), European Regional Development Fund (FEDER) and the Carlos III Health Institute (PI11/00667, PI14/00707 and PI17/00384).
Received: March 17, 2020; Accepted: April 08, 2020











 texto em
texto em