My SciELO
Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista de Osteoporosis y Metabolismo Mineral
On-line version ISSN 2173-2345Print version ISSN 1889-836X
Rev Osteoporos Metab Miner vol.12 n.4 Madrid Oct./Dec. 2020 Epub Apr 05, 2021
https://dx.doi.org/10.4321/s1889-836x2020000400004
ORIGINALS
Agitated/inattentive children: explanatory models1
1Department of Rheumatology. La Paz Hospital. Health Research Institute of the La Paz University Hospital (IdiPAZ). Madrid (Spain)
2Department of Nuclear Medicine. La Paz Hospital. Madrid (Spain)
3Galgo Medical. Barcelona (Spain)
Objetivo
Evaluar la densidad mineral ósea (DMO) y parámetros de 3D-Shaper a nivel de fémur proximal (FP) en adultos con hipofosfatasia (HPP) confirmada genéticamente y compararlos en aquellos sujetos con y sin fracturas.
Material and methods
Análisis transversal de datos densitométricos y de arquitectura ósea de la visita basal de un estudio longitudinal en el que se incluyeron pacientes con HPP. Se realizó un estudio densitométrico (Lunar Prodigy, GE iDXA) en FP y se empleó el software 3D-Shaper (version 2,7. Galgo Medical).
Results
Se incluyeron 33 adultos con HPP con mutaciones en heterocigosis. Un 63,6% (21/33) fueron mujeres (42,9% postmenopáusicas), y 8 de los varones (66,6%) fueron mayores de 50 años. La media de edad fue 50,56±15,08 años, el 30,3% (10/33) tuvieron fracturas previas traumáticas, y un 15,2% (5/33), de estrés. La prevalencia de osteoporosis en CF fue del 11,8% (2/17) y de osteopenia, 82,4% (14/17). En premenopáusicas y varones jóvenes se detectó baja masa ósea para la edad en un 12,5% (2/16). Al comparar sujetos con fracturas de estrés y sin ellas, así como con traumáticas, no hubo diferencias en DMO. El 3D-Shaper mostró disminución del grosor cortical (mm) en pacientes con fracturas de estrés [1,8 (1,77-1,89)] frente a sujetos sin ellas [1,94 (1,87-2,03, p=0,03)] y en comparación con los que tuvieron fracturas traumáticas [1,97 (1,88-2,04), p=0,03].
Conclusions
Estos datos reflejan una discreta repercusión densitométrica en formas más leves del adulto. Estudios de arquitectura ósea pudieran resultar de interés para determinar pacientes susceptibles de presentar fracturas de estrés.
Key words osteoporosis; hypophosphatasia; bone densitometry; 3D-DXA
INTRODUCTION
Hypophosphatasia (HPP) is a rare metabolic disease characterized by low enzymatic activity of non-tissue-specific alkaline phosphatase (TNSALP), which causes an accumulation of its natural substrates: inorganic pyrophosphate (PPi), pyridoxal-5'-phosphate (PLP) and phosphoethanolamine (PEA)1. PPi acts as a potent inhibitor of hydroxyapatite crystal formation and its high extracellular levels can induce skeletal alterations, such as decreased bone mineralization2,3. In general, the more severe forms are associated with earlier symptoms and diagnosis, even perinatal, while the milder forms often present later in childhood or adulthood4. The importance of an early diagnosis lies in the potential severity of the disease and the alteration of the quality of life, as well as in the possible iatrogenesis derived from a wrong diagnosis and treatment5. Previous studies have analyzed the symptoms that characterize adult HPP, which usually shows a wide range of clinical manifestations, sometimes nonspecific, such as the presence of musculoskeletal pain, weakness, dental pathology or early loss of teeth, and the presence of of recurrent stress fractures and pseudofractures6,7. In a pediatric age cohort, the analysis of bone mineral density (BMD) in these patients has detected low values in the most severe cases8.
However, the limited evidence available in adults with HPP has shown a normal or slightly decreased BMD7,9-12, from which it is deduced that bone densitometry may not adequately predict the risk of fracture7. Therefore, the objective of this study is to evaluate the BMD in the proximal femur (PF) and carry out a volumetric analysis of the cortical and trabecular bone of this region, as well as cortical thickness using 3D-Shaper in subjects with HPP, and compare these parameters between subjects with and without a history of fractures.
MATERIAL AND METHODS
Study population and design First, a search was carried out in the biochemical database of our tertiary hospital and associated outpatient clinics in which 383,353 patients with alkaline phosphatase (APH) determinations were located, of which 231,805 were adults with at least two measurements. Of these, 427 showed persistent hypophosphatasemia (≥2 determinations less than or equal to 35 IU/L and none greater than 45 IU/L; normal range: 46-116 IU/L). Subsequently, their medical records were reviewed, and 31 subjects were excluded due to underlying secondary causes of hypophosphatasemia13 and 13 due to the impossibility of telephone contact. A total of 383 subjects met the selection criteria and were contacted, of which 85 signed the informed consent for the performance of a genetic test to detect variants in the ALPL gene. 39 (46%) patients with pathogenic or probably pathogenic mutations were detected and offered follow-up in our consultations.
This paper presents the cross-sectional analysis of the baseline densitometric and bone architecture data of 33 adults subsequently included in a prospective observational longitudinal study carried out at Hospital La Paz (Madrid). Details related to the recruitment process have been reported in a previous publication by our group14. The study has been approved by the Ethics and Research Committee of Hospital La Paz. Informed consent has been obtained from the patients.
METHODS
They were collected using a protocolized questionnaire and risk factors for osteoporosis were analyzed, including smoking, alcohol intake (≥30 g/day), sun exposure (≥10 minutes/day), exercise practice, dietary calcium intake from dairy products (1 serving = 1 glass of milk = 2 yogurts = 1 serving of cheese (40-50 mg) = 200 mg/calcium), personal history of fracture and its etiology, as well as family history of hip fracture.
A densitometric study (Lunar Prodigy, General Electric Medical Systems iDXA) was performed in each of the subjects for the analysis of BMD in FP (neck, trochanter, total hip and femoral shaft). The presence of osteoporosis has been defined according to the WHO criteria15. On the other hand, the 3D-Shaper software (version 2.7, Galgo Medical) was used to evaluate the volumetric density of the cortical and trabecular bone of the FP. The software uses a 3D statistical model of the FP and adjusts it on the densitometric image, to achieve a personalized 3D model of the shape and distribution of the bone BMD. The measures provided by the software include volumetric bone mineral density (BMD) of the cortical, trabecular, and integral compartments, cortical superficial bone mineral density (BMD), and cortical thickness. Additional information on the methodology implemented in the software and its validation can be found in previous works16.
Statistic analysis
For the description of the sample, the mean and standard deviation (SD) or median and interquartile range (IQR) were calculated for the quantitative variables, as well as the absolute number and the relative percentages for the qualitative variables in each group. Comparisons between groups were made using the U-Mann-Whitney statistical test. All analyzes were performed using the SPSS version 23.0 statistical package for Windows.
RESULTS
Demographic, clinical, densitometric and 3D-Shaper data of subjects with HPP
Thirty-three adults with HPP were included, all of whom had pathogenic or probably pathogenic variants in heterozygosity. 63.6% of the patients (21/33) were women (42.9% postmenopausal), and 8/12 of the men were older than 50 years (66.6%). The mean age was 50.56±15.08 years; BMI, 26.31±4.39 kg/m2, and mean alkaline phosphatase, 25.2±6.53 IU/L. 12.1% (4/33) had a family history of hip fracture. The total number of fractures was 16: 30.3% (10/33) had a personal history of fractures of traumatic etiology (3 in the metatarsals, 4 in the hand bones, 2 in the elbow and one in the clavicle) and 15.2% (5/33), previous stress fractures (three patients in one metatarsal, one patient in two metatarsals and one patient, atypical femoral shaft fracture that meets the criteria of the American Society for Bone and Mineral Research (ASBMR)17, without previous exposure to diphosphonates. No patient had fragility fractures.
The demographic characteristics and the rest of the risk factors for osteoporosis are described in table 1. The prevalence of osteoporosis in the femoral neck was 11.8% (2/17), and osteopenia was detected in 82.4% (14/17) of patients. In premenopausal women and men under 50 years of age, low bone mass was observed for the age range in 12.5% ( 2/16) of patients. The rest did not show alterations in the densitometric study of the femoral neck (FN). The mean BMD in the femoral neck of the patients with osteoporosis was 0.73±0.01 and the T-score was -2.65±0.7. In patients with osteopenia, the mean BMD in this region was 0.86±0.05 g/cm2 and the T-score was -1.3±0.43. In subjects with low bone mass for their age range, the mean BMD was 0.67±0.07 g/cm2 and the Z-score was -2.4±0.6.
Tabla 1. Demographic characteristics and risk factors for osteoporosis of patients with HPP
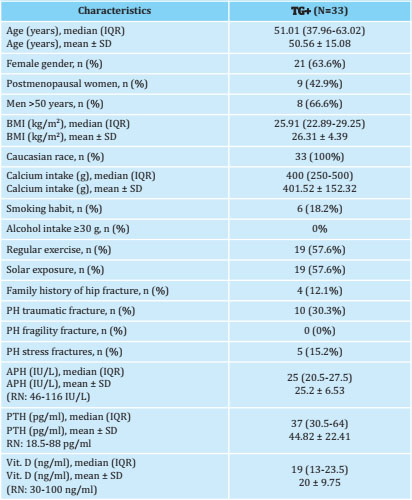
Quantitative data are expressed as median (interquartile range, IQR) and mean ± standard deviation (SD). The qualitative ones as absolute numbers and percentages. p<0.05 is considered significant. TG+: patients with persistent hypophosphatasemia and a positive genetic test confirming hypophosphatasia; BMI: body mass index; PH: personal history; APH: alkaline phosphatase; PTH: parathyroid hormone; Vit. D: vitamin D; NR: normal range.
In relation to stress fractures, two postmenopausal patients had both femoral diaphysis and metatarsal fractures, and both had bone densitometry in the osteopenic range, while the three premenopausal patients with metatarsal fractures had normal densitometry. Among the patients with traumatic fracture, five were men (four aged >50 years). One of them suffered a traumatic elbow fracture and presented osteoporosis in CF, while the other three suffered metatarsal, elbow and scaphoid fractures, and presented osteopenia. The patient under 50 years old had a traumatic fracture in a metacarpal and normal bone densitometry.
The median BMD at the CF level was 0.876 (0.83-0.92) g/cm2; at the level of the femoral shaft, 1.123 (1.03-1.21) g/cm2; 0.759 (0.693-0.82) g/cm2in the trochanteric region, and 0.931 (0.89-1) g/cm2in the total hip. The Z-score value at the CF level was -0.29 (-1.13-0.25) and in total hip, -0.06 (-0.79-0.39). In the 3D-Shaper analysis, a total cortical BMD of 813.45 (759.12-862.26) mg/cm3 was observed, a trabecular BMD of 155.77 (136.73-180.08) mg/cm3, a total cortical BMD of 157.55 (144.8-166.94) mg/cm2 and a total integral BMD of 309.29 (280.3-324.62) mg/cm3. Cortical thickness (mm) was 1.89 (1.85-2.01). Table 2 includes densitometric data and 3D-Shaper parameters of patients with HPP.
Tabla 2. Densitometry and 3D-Shaper data in patients with HPP

Data are expressed as median (interquartile range, IQR). TG+: patients with persistent hypophosphatasemia and a positive genetic test confirming hypophosphatasia; BMD: bone mineral density; VBMD: volumetric bone mineral density; SBMD: superficial bone mineral density; p<0.05 is considered statistically significant.
Demographic, clinical, densitometric and 3D-Shaper data of subjects with PPH with fracture versus without fracture
In the first place, the comparison of demographic, clinical, densitometric and 3D-Shaper data of the five subjects with HPP who presented stress fractures compared to the 28 who did not. The patients with stress fractures were women, 40% postmenopausal, with a mean age of 46.35±10.1 years, while in the group of patients without them, 57.14% were women (25% postmenopausal ) with a mean age of 51.31±15.82 years (p=0.48). Differences were observed in the prevalence of women, higher in the first group (p=0.07) and in BMI, lower in patients with stress fractures (23.5±2.44 kg/cm2) compared to the other group (26.81±4.5 kg/cm2; p=0.07). There were no differences in the rest of the risk factors for osteoporosis. Although no differences were observed in BMD parameters in FP, the 3D-Shaper analysis showed a statistically significant difference in cortical thickness (mm), which was less in those patients with stress fractures [1,8 (1 .77-1.89)] versus the non-fractured [1.94 (1.87-2.03); p=0.03)].
Second, the 9 patients with a history of traumatic fracture were compared to the 19 without a history of fracture, and no densitometric differences were observed or in 3D-Shaper parameters (p>0.05). Third, in the comparison between subjects with stress fractures and those who presented fractures of traumatic etiology, a decrease in cortical thickness (mm) was observed, lower in patients in the first group [1.8 (1.771.89)] versus the second [1.97 (1.88-2.03), p=0.03]. No differences were observed in the rest of the densitometric parameters or DXA-3D.
From a biochemical point of view, there were no significant differences in alkaline phosphatase, PTH and vitamin D levels between groups (p>0.05). Table 3 shows the intergroup comparison of demographic, clinical, densitometric and 3D-Shaper data, and figure 1 shows the levels of AF stratified by groups.
Tabla 3. Demographic, clinical, densitometric and 3D-Shaper data of subjects with versus without fracture

Quantitative data are expressed as mean and standard deviation (SD) and median, interquartile range (IQR) and qualitative data as frequencies and percentages. BMI: body mass index; FH: family history; APH: alkaline phosphatase; PTH: parathyroid hormone; vit. D: vitamin D; BMD: bone mineral density; VBMD: volumetric bone mineral density; SBMD: superficial bone mineral density; p<0.05 is considered statistically significant.
DISCUSSION
In this work, a descriptive analysis of the densitometric characteristics of adults with HPP and of the bone architecture parameters was carried out using 3D-Shaper, a technique still little explored in these patients, as well as a comparison of these parameters between patients with and without a history of fractures.
In our study we found that densitometric alterations were not particularly relevant in terms of the prevalence of osteoporosis. The repercussion has resulted in more moderate changes in these patients, with a high prevalence of osteopenia, more pronounced in postmenopausal women and men over 50 years of age. The slight decrease in BMD observed in this study seems to be in accordance with the results found in the existing literature on adults with HPP, in which the majority of patients presented normal parameters or a slight decrease in Z-score values, findings that seem respond to milder forms of the adult5,7,9.
Previous studies have not found differences in BMD in subjects diagnosed with HPP with and without fractures, suggesting that this test may not adequately translate the risk of presenting them7. Nor in our study have we found significant differences in the densitometric analysis between individuals with HPP with and without a history of stress fractures. However, the 3D-Shaper technique shows a statistically significant decrease in cortical thickness (mm) at the FP level in patients with stress fractures [1.8 (1.77-1.89)] compared to those without this history. [1.94 (1.87-2.03, p=0.03)] and compared to those with traumatic fractures [1.97 (1.88-2.04), p=0.03] that does not seem to be explained by a lower level of alkaline phosphatase.
In this same disease, applying high-resolution peripheral quantitative computed tomography (HR-pQCT) in the left distal tibia and right distal radius, Schmidt et al. also reported a decrease in cortical thickness in patients with HPP with fractures compared to those without fractures7. Likewise, other work has highlighted the presence of a decrease in cortical thickness in the radiographs of some adult patients with HPP18.
Although we have not found evidence from other studies that analyze 3D-Shaper parameters in HPP, other publications have evaluated this technology in patients with different bone metabolic pathologies. Gracia-Marco et al.19 observed differences in cortical thickness in subjects with primary hyperparathyroidism, lower in patients with this disease compared to healthy controls (1.85±0.14 mm vs. 1.93±0.17 mm ; p=0.023). These results suggest that bone architecture studies could be of special interest with other diseases involving high bone remodeling. However, Humbert et al.20 observed a non-significant decrease in cortical thickness in postmenopausal patients with hip fracture compared to controls (1.746±0.127 mm vs. 1.783±0.123 mm; p=0.1). In our study, patients with HPP and traumatic fracture did not show a decrease in cortical thickness compared to those who did not fracture.
Stress fractures were originally described in military recruits and were considered “fatigue fractures” as a consequence of repeated and prolonged minimal or small mechanical impacts on a bone with normal elastic resistance. A subtype of stress fractures is insufficiency fractures produced by a normal load on a bone with altered resistance, described in patients with vitamin D deficiency (Looser-Milkman lines, characteristic of osteomalacia)21,22. A high prevalence of insufficiency fractures or pseudofractures has also been described in patients with HPP1, but we do not know exactly which patients will develop them. Regarding their location, recurrent metatarsal fractures and femoral fractures and pseudofractures are characteristic, which are those found in our patients.
Alkaline phosphatase levels expressed as median (interquartile range) stratified by groups. FA: alkaline phosphatase; Fx: fracture.
With probable multifactorial pathogenesis, stress fractures could reflect alterations in BMD and bone quality23. In our study, we did not find differences in BMD at the PF level of patients who had stress fractures versus those who did not, coinciding with what was recently published by other authors11. The decrease in cortical thickness observed in our patients with stress fractures would reinforce the existence of a qualitative bone alteration. López Delgado et al.12 describe low bone remodeling in patients with persistent hypophosphatasemia, although this does not seem to translate into differences in BMD or trabecular bone score (TBS) when compared with a control group. Our patients with stress fractures did not show differences in the level of alkaline phosphatase decrease compared to those who do not fracture, so it is difficult to explain the presence of fractures due to a greater severity of the enzyme defect.
As limitations of our study, most of our subjects present heterozygous mutations that condition milder forms of the disease. From the densitometric point of view, that, up to now, we do not have the reference population values for 3D-Shaper measurements, they have not been compared with a control group, a fact that may limit how the results are interpreted. As strengths, however, it is worth highlighting the significant number of patients, given that this is a rare disease and studied using a new technique.
These data seem to reflect a discrete impact at the densitometric level in the mildest adult forms. A decrease in cortical thickness was identified in patients with HPP with stress fractures. Bone architecture studies in PF could be of interest to determine subjects with HPP susceptible to presenting this type of fracture.
Ethics Committee approval: All the studies carried out followed the principles set forth in the Helsinki declaration and were formally approved by the La Paz Hospital Clinical Trials Committee (PI 3239). Informed consent has been obtained from all patients.
REFERENCES
1 Whyte MP. Hypophosphatasia - aetiology, nosology, pathogenesis, diagnosis and treatment. Nat Rev Endocrinol. 2016;12(4):233-46. [ Links ]
2 Caswell AM, Whyte MP, Russell RG. Hypophosphatasia and the extracellular metabolism of inorganic pyrophosphate: clinical and laboratory aspects. Crit Rev Clin Lab Sci. 1991;28(3):175-232. [ Links ]
3 Millán JL, Whyte MP. Alkaline phosphatase and hypophosphatasia. Calcif Tissue Int. 2016;98(4):398-416. [ Links ]
4 Lefever E, Witters P, Gielen E, Vanclooster A, Meersseman W, Morava E, et al. Hypophosphatasia in adults: clinical spectrum and its association with genetics and metabolic substrates. J Clin Densitom. 2020;23(3):340-8. [ Links ]
5 Martos-Moreno GA, Calzada J, Couce ML, Argente J,. Hipofosfatasia: manifestaciones clínicas, recomendaciones diagnósticas y opciones terapéuticas [Hypophosphatasia: Clinical manifestations, diagnostic recommendations and therapeutic options]. An Pediatr (Barc). 2018;88(6):356. [ Links ]
6 Berkseth KE, Tebben PJ, Drake MT, Hefferan TE, Jewison DE, Wermers RA. Clinical spectrum of hypophosphatasia diagnosed in adults. Bone. 2013; 54(1):21-7. [ Links ]
7 Schmidt T, Mussawy H, Rolvien T, Hawellek T, Hubert J, Rüther W, et al. Clinical, radiographic and biochemical characteristics of adult hypophosphatasia. Osteoporos Int. 2017;28(9):2653-62. [ Links ]
8 Whyte MP, Zhang F, Wenkert D, McAlister WH, Mack KE, Benigno MC, et al. Hypophosphatasia: Validation and expansion of the clinical nosology for children from 25years experience with 173 pediatric patients. Bone. 2015;75:229-39. [ Links ]
9 Barvencik F, Timo Beil F, Gebauer M, Busse B, Koehne T, Seitz S, et al. Skeletal mineralization defects in adult hypophosphatasia - A clinical and histological analysis. Osteoporos Int. 2011;22(10):2667-75. [ Links ]
10 Wüster C, Ziegler R. Reduced bone mineral density and low parathyroid hormone levels in patients with the adult form of hypophosphatasia. Clin Investig. 1992;70(7):560-5. [ Links ]
11 Genest F, Claußen L, Rak D, Seefried L. Bone mineral density and fracture risk in adult patients with hypophosphatasia. Osteoporos Int. 2021;32(2):377-85. [ Links ]
12 López-Delgado L, Riancho-Zarrabeitia L, García-Unzueta MT, Tenorio JA, García-Hoyos M, Lapunzina P, et al. Abnormal bone turnover in individuals with low serum alkaline phosphatase. Osteoporos Int. 2018;29(9):2147-50. [ Links ]
13 McKiernan FE, Berg RL, Fuehrer J. Clinical and radiographic findings in adults with persistent hypophosphatasemia. J Bone Miner Res. 2014;29(7): 1651-60. [ Links ]
14 Tornero C, Navarro-Compán V, Tenorio JA, García-Carazo S, Buño A, Monjo I, et al. Can we identify individuals with an ALPL variant in adults with persistent hypophosphatasaemia? Orphanet J Rare Dis. 2020;15(1):51. [ Links ]
15 Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. Report of a WHO Study Group. World Health Organ Tech Rep Ser. 1994;843:1-129. [ Links ]
16 Humbert L, Martelli Y, Fonolla R, Steghofer M, DI Gregorio S, Malouf J, et al. 3D-DXA: Assessing the femoral shape, the trabecular macrostructure and the cortex in 3D from DXA images. IEEE Trans Med Imaging. 2017;36(1):27-39. [ Links ]
17 Shane E, Burr D, Abrahamsen B, Adler RA, Brown TD, Cheung AM, et al. Atypical subtrochanteric and diaphyseal femoral fractures: second report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2014;29(1):1-23. [ Links ]
18 Whyte MP, Teitelbaum SL, Murphy WA, Bergfeld MA, Avioli LV. Adult hypophosphatasia. Clinical, laboratory, and genetic investigation of a large kindred with review of the literature. Medicine (Baltimore). 1979; 58(5):329-47. [ Links ]
19 Gracia-Marco L, García-Fontana B, Ubago-Guisado E, Vlachopoulos D, García-Martín A, Muñoz-Torres M. Analysis of bone impairment by 3D DXA hip measures in patients with primary hyperparathyroidism: a pilot study. J Clin Endocrinol Metab. 2020; 105(1): dgz060. [ Links ]
20 Humbert L, Bagué A, Di Gregorio S, Winzenrieth R, Sevillano X, González Ballester MÁ, et al. DXA-based 3D analysis of the cortical and trabecular bone of hip fracture postmenopausal women: a case-control study. J Clin Densitom. 2020;23(3):403-10. [ Links ]
21 Matcuk GR Jr, Mahanty SR, Skalski MR, Patel DB, White EA, Gottsegen CJ. Stress fractures: pathophysiology, clinical presentation, imaging features, and treatment options. Emerg Radiol. 2016;23(4):365-75. [ Links ]
22 Anguita Martínez G, Vega González ML, Cobos Huerga C, Moreno Casado MJ. Fracturas de estrés de los metatarsianos. Rev Int Cienc Podol. 2011;5 (2):47-54. [ Links ]
23 Moreira CA, Bilezikian JP. Stress fractures: concepts and therapeutics. J Clin Endocrinol Metab. 2017;102(2):525-34. [ Links ]
Received: November 12, 2020; Accepted: January 21, 2021











 text in
text in 



