My SciELO
Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista de Osteoporosis y Metabolismo Mineral
On-line version ISSN 2173-2345Print version ISSN 1889-836X
Rev Osteoporos Metab Miner vol.15 n.1 Madrid Jan./Mar. 2023 Epub May 29, 2023
https://dx.doi.org/10.20960/revosteoporosmetabminer.00006
ORIGINALS
Implication of connexins, integrins and primary cilium in bone cell activity
3Institute of Applied Medicine of the Universidad San Pablo-CEU. Madrid, Spain
4Department of Basic Medical Sciences. School of Medicine. Universidad San Pablo-CEU. Madrid, Spain
Background:
osteocytes are capable of detecting different signals, transducing them into biological responses and transmitting them to osteoblasts and osteoclasts, allowing the maintenance of bone homeostasis. Bone mechanotransduction is possible because osteocytes have different mechanosensor structures such as connexins (Cxs), integrins, the primary cilium and even receptors coupled to G proteins such as the type 1 parathyroid hormone receptor (PTH1R).
Objective:
to analyze the possible interaction of the different mechanosensor elements of the osteocytes and to observe their influence on the biological response.
Material and methods:
we worked with the osteocytic cell lines MLO-Y4 Cx43+/+ (scrambled [SCR] and RNAi α2) and Cx43-/-.
Results and conclusion:
our results show that Cx43 and integrin α2 are involved in lengthening the primary cilium, potentially affecting its functionality as a mechanosensor (SCR vs RNAi α2, p < 0.0001 SCR vs Cx43-/- and p < 0.0001 RNAi α2 vs Cx43-/-). The α2 integrin also influenced the cellular localization of Cx43, promoting its presence in the plasma membrane. Activation of PTH1R by agonists such as parathyroid hormone (PTH) and parathyroid hormone-related protein (PTHrP) was also found to induce ERK 1/2 kinase phosphorylation, and these effects could be affected by Cx43 deficiency, but do not appear to be. mediated by the silencing of α2 integrin. Finally, it was observed that the presence of Cx43 and integrin α2 in osteocytes increases their adhesion capacity (Cx43+/+ SCR and RNAi α2 vs CX43-/- p < 0.001 and p = 0.0039) and that deficiency in Cx43 causes an increase in the mortality of these cells (Cx43-/- vs Cx43+/+ p = 0.0074).
Keywords: Osteocytes; Connexin 43; Primary cilium; Integrins; Mechanical stimulation; PTHrP
INTRODUCTION
Bone is a dynamic tissue that constantly remodels itself in response to a wide variety of stimuli, including hormones, growth factors and mechanical loading (1). Precise, coordinated control of bone remodeling requires interaction and communication between osteoblasts (bone-forming cells), osteocytes (main bone mechanosensor cells) and osteoclasts (cells responsible for bone resorption). It takes place, among other mechanisms, thanks to the formation of gap junctions (GJ) between these bone cells (2).
Osteocytes are capable of detecting mechanical stimuli due to the fact that they present different mechanosensory structures: integrins, GJ, connexin 43 hemichannels (Cx43), primary cilia and/or G protein-coupled receptors (GPCR), such as PTH1R (3). Subsequently, these cells transduce the mechanical stimuli into biological responses, which trigger the activation of different signaling pathways, inducing changes in gene expression and cell metabolism. This causes the secretion of factors capable of regulating the proliferation and viability of bone effector cells (osteoblasts and osteoclasts). Due to the complexity of the extracellular environment of bone, it is very likely that the different mechanosensors interact with each other, integrating the multiple extracellular signals into a cohesive signal (4).
Cellular communication plays an important role in bone tissue, being embedded inside a mineralized matrix (5). Connexins (Cxs) are proteins that constitute some of the essential channels for communication between bone cells to take place.
Osteocytes may also promote bone formation due to the endocrine actions of PTH and its local analogue in bone, PTHrP, through the activation of PTH1R, their common receptor (6). PTH1R is a GPCR that can trigger various intracellular signaling pathways in bones (7).
The primary cilium is a mechanosensor structure capable of creating a different microdomain of the cell cytoplasm. This allows the specific location and concentration of receptors such as GPCRs, ion channels and effector proteins, thereby improving the kinetics of signaling pathways (8).
Various studies have shown that defects in the function or sensory structure of the primary cilium are associated with different diseases, generally referred to as ciliopathies (9). Likewise, when the formation of primary cilia is interrupted or their length decreases, the cells present an altered mechanosensitivity and a diminished response to mechanical stimulation (10).
Integrins are protein complexes that allow the cell to interact with the extracellular environment (11). Previous research has shown that in MLO-Y4 cells, β1 and α2 integrins are involved in the activation of extracellular signal-regulated kinases (ERK 1/2), induced by mechanical stimuli, which leads to the activation of signaling pathways that modulate the adhesion of osteocytes to the mineralized matrix and inhibit the apoptotic response of these cells (12).
In this study, we hypothesized the possible relationship between connexins, primary cilia, PTH1R and integrins as regulators of biological processes that would be crucial to the function of osteocytes.
MATERIALS AND METHODS
CELL CULTURE
Three types of MLO-Y4 cells Cx43+/+ (presenting an empty vector as a negative control to be able to evaluate the effects of non-silencing) and Cx43-/- (cells deficient in connexin 43, transfected with an RNAi) were used, which were kindly provided by Dr. L. I. Plotkin, and Cx43+/+ cells in which α2 integrin was silenced by RNAi. These cells were seeded at a concentration of 24,000 cells/cm2 and cultured with α-Modified Eagle’s Medium (α-MEM) (Gibco, ThermoFisher Scientific, ES) supplemented with 2.5 % calf serum (Calf Serum; CS), 2.5 % fetal bovine serum (Fetal Bovine Serum; FBS), 1 % L-glutamine, 1 % penicillin/streptomycin and Puromycin from Streptomyces alboniger (Sigma Aldrich, ES) at a concentration of 10 μg/ml.
All the surfaces on which these cells were seeded had to be previously collagenized with type I collagen at 0.01 % acetic acid13. Cells were kept at 37 °C and 5 % CO2.
SILENCING OF α2 INTEGRIN
MLO-Y4 Cx43+/+ cells were transfected with three different α2 silencers (RNAi) (5 nM) (ThermoFisher Scientific, ES), targeting the α2 integrin sequence, using lipofectamine RNAiMax (Life Technologies, Carlsbad, CA, USA). The siRNAs were added in serum-free medium for 24 h. The scrambled sequence (SCR) (RNAi control, Santa Cruz Technology, TX, USA) was used as a negative control to evaluate the non-targeted effects of silencing (RNAi off-targeted).
PCR
RNA extraction was performed with TRIZOL® (Ambion, FosterCity, CA, USA). For RNA reverse transcription (RTPCR, reverse transcriptase polymerase chain reaction) the kit (Applied Byosistems, Grand Island, NY, USA) and the thermocycler (Eppendorf, Hamburg, Germany) were used.
To analyze the expression of the α2 integrin (Fw 5´CCATGATGGGTCGAAGCTGA3´; Rv 5´CTTCGTCGGCCACATTGAAA3´) SYBR Green (Sybr promix ex Taq, Takara, Otsu, Japan) was used. The level of α2 integrin expression was analyzed using β-actin as control gene (Fw 5´GAACCCTAAGGCCAACCGTG3´; Rv 5´ACCAGAGGCATACAGGGACAG3´). Triplicates of all conditions (Cx43+/+ and Cx43-/-) were performed. The expression change of the genes was calculated based on the value of 2-ΔΔCt.
IMMUNOFLUORESCENCE
Multiwell plates (Falcon, ES) were seeded at 30,000 cells/well. Cells were grown until they reached 80 % confluency and serum-free medium was added for 24 h to induce primary cilium formation. Cells were then fixed with 4 % paraformaldehyde (PFA) and permeabilized with 0.5 % Triton X-100. Next, the blocking solution composed of 10 % bovine serum albumin (BSA), supplemented with 5 % goat serum, was added for 1 h. Subsequently, the following primary antibodies were kept overnight at 4 °C under agitation: polyclonal anti-Cx43 produced in rabbit (Sigma, ST. Louis, MO, USA) (1:1000 dilution in 10 % BSA and 5 % goat serum); and mouse monoclonal acetylated anti-α tubulin (Sigma) (1:1000 dilution in 10 % BSA and 5 % goat serum), to observe the primary cilium. Secondary antibodies were then added: Alexa fluor® 488 goat anti-mouse primary cilia (Invitrogen Molecular probes, Thermo Fisher ScientificTM, ES) (1:1000 dilution in 10 % BSA and 5 % goat serum), to Cx43 Alexa fluor® 568 anti-rabbit IgG (Live technologies, Thermo Scientific™, ES) (1:1000 dilution in 10 % BSA and 5 % goat serum). After 1 h of incubation, 4’-6-diamidino2-phenylindole (DAPI) (1:10,000 dilution) was added. The nuclei, the primary cilium and the Cx43 were visualized with the epifluorescence microscope (Leica CTR 6000). Triplicates were imaged and a total of 100 cells from each condition (Cx43+/+ SCR and α2 and Cx43-/-) were analyzed. The fusion of the individual images of the primary cilium, Cx43 and cell nuclei into one was performed with the ImageJ program, which allows processing digital images, is capable of calculating the area and the statistics of the value of the pixel selected by the user and to measure distances.
STIMULATION BY PTH1R AGONISTS (PTH AND PTHRP)
To perform stimulation by PTHrP and PTH, cells were plated at a density of 25,000 cells/cm2 and both ligands were added at a concentration of 10-7 molar (M), for 10 min.
CELL DEATH AND ADHESION ASSAY
Cell viability was determined by trypan blue exclusion, a method that stains dead cells blue, allowing the percentage of live and dead cells to be calculated with respect to the total using a Neubauer camera and a bright field optical microscope (Leica DM5500B). to do the counting.
After trypsinizing the cells and 30 minutes after reseeding, non-adherent cells were counted with the Neubauer chamber. Next, images of different fields of the cells adhered to the Petri dish were taken, using an inverted bright field microscope (Leica DM5500B), to calculate the percentages of cells adhered to the total. A total of 9 fields of each condition were analyzed in triplicate (Cx43+/+ SCR and α2 and Cx43-/-).
WESTERN BLOT ANALYSIS
Protein extraction was performed for each condition in duplicate (Cx43+/+ SCR and α2 and Cx43-/-), using RIPA Buffer (150 mM NaCl, 1.0 % IGEPAL® CA-630, 0.1 % SDS (sodium dodecyl sulfate), 50 mM Tris, pH 8, Sigma-Aldrich, St Louis, MO, USA), protease inhibitor (PI) (1:100 dilution, Calbiochem®, ES) and phosphatase inhibitor (IF) (1:100 dilution, Calbiochem®, IT IS). Proteins were quantified by the bicinchoninic acid (BCA) assay (Pierce™ BCA Protein Assay Kit, Thermo Fisher Scientific, ES). To carry out the reading, the Variouskan Flash plate reader (Thermo Fisher Scientific, ES) was used and three readings were made at 562 nm using the SkanIt Software 2.4.3 RE program.
Protein extracts were separated using a polyacrylamide gel. Proteins were subsequently transferred to a nitrocellulose membrane (Bio-Rad, Hercules, CA, USA). The blocking was carried out with 5 % milk powder in Tris saline buffer with Tween20 (TTBS) at 0.05 %, for 1 h under stirring at room temperature. Next, the following primary antibodies were incubated for 24 hours at 4 °C and under agitation: anti-phospho-p44/42 MAPK (Erk 1/2) (Cell Signaling, Beverly, MA, USA), anti-p44/42 MAPK (Erk 1/2), and anti-α-tubulin (Sigma Aldrich, ES). Subsequently, secondary antibodies were added. Chemiluminescence development was carried out with ClarityTM Western ECL substrate (Bio-Rad). The intensity of the bands was quantified by densitometry, using Dnr Bio Imaging System MF ChemiBIS3.2 and the programs Gelcapture and QuantityOneTM (Bio-Rad).
STATISTICAL ANALYSIS
The confidence limit established in all the statistical tests was 95 %. Therefore, results with a p value (p) p < 0.05 are considered statistically significant.
For the comparison of means ± standard deviation (SD), the GraphPad Prism 8 program was used. To compare means of more than two groups, the ANOVA test of a single factor and the ANOVA Welch test were used. Equality of variances was not considered. For multiple analyses, Dunnett’s test, Tukey’s test and the non-parametric Kruskal Wallis test were used. Being t the statistic that analyzes if the measures of the two conditions are equal or not and gl that would be the degrees of freedom that indicate the number of values that can be assigned arbitrarily.
RESULTS
INTEGRIN GENE EXPRESSION MODULATED BY CONNEXIN 43 IN OSTEOCYTIC CELLS
The RNA expression of integrins α2, α6, β1, β3 and β6 and Cx43 was analyzed by means of quantitative PCR, to study the possible relationship in the expression of these two families of proteins.
Figure 1 shows how the α2 integrin significantly decreases its expression level in cells of the Cx43-/- line compared to Cx43+/+ cells (t = 13.93, gl = 4, p = 0.0002). On the contrary, the α6, β1 and β3 integrins significantly increase their expression in the Cx43-/- cell line compared to Cx43+/+ (t = 3.646, df = 4, p = 0.0219; t = 5.501, g = 4, p = 0.0053, t = 26.18, gl = 4, p < 0.0001, respectively). In the case of β6, no significant differences were observed between the two cell lines (t = 0.99, df = 4, p = 0.378).

Figure 1. RNA expression of integrins α2, α6, β1, β3 and β6 in Cx43 +/+ and Cx43-/- cells. The results are expressed as mean ± SD of an experiment performing triplicates of each experimental condition *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001.
These results indicate that the expression of connexin 43 conditions the expression pattern of various integrins in osteocytic cells.
CONNEXIN 43 AND INTEGRIN α2 REGULATE THE LENGTH OF THE PRIMARY CILIUM IN OSTEOCYTIC CELLS
Immunofluorescence was performed to observe the possible relationship and interaction between the primary cilium, Cx43 and α2 integrin, three well-known mechanosensors of osteocytes, and to determine if the development and length of the primary cilium could depend on the presence of Cx43 and α2 integrin (Fig. 2).
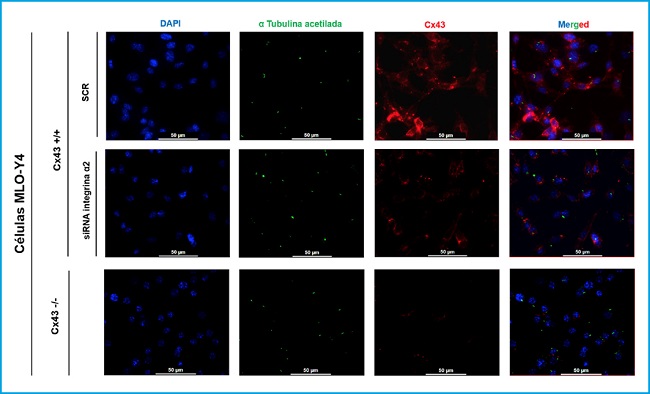
Figure 2. Immunofluorescence of MLO-Y4 Cx43+/+ (SCR and RNA α2) and Cx43 -/- cells. The cell nucleus was visualized with DAPI (blue), the primary cilium with the anti-α-acetylated tubulin antibody (green) and the Cx43 with the anti-Cx43 antibody (red). Images were captured with a confocal fluorescence microscope (40X). Scale bar = 50 μm.
MLO-Y4 Cx43+/+, MLO-Y4 Cx43+/+ cells silenced with α2 integrin (RNAi α2), and MLO-Y4 Cx43-/- cells were compared. In the results shown in figure 3, it was observed that all cell lines are capable of developing primary cilium and that this organelle originates on the cell surface. In addition, it was observed that Cx43 presents a different distribution in Cx43+/+ and α2 RNAi cells, since in cells in which the α2 integrin was not silenced, the presence of Cx43 predominates in the cell membrane, an expected location, since it is where it forms hemichannels and UCs. While in the conditions in which the cells do not present α2 integrin, Cx43 is distributed in the cell cytoplasm and not so focused on the plasma membrane. Immunofluorescence images also showed that Cx43 and the primary cilium do not co-localize.

Figure 3. Comparison of primary cilium length (μm) between lines Cx43+/+ (SCR and RNAi α2) and Cx43-/-. A. The results show the mean value ± SD of the length of the primary cilium in mean μm from the images made at different fields. *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001. B. Representative images in which the nucleus can be visualized by DAPI (blue) and the primary cilium by anti-α-acetylated tubulin antibody (green). The images were captured with a confocal fluorescence microscope (40X). Scale bar = 50 μm.
In order to quantitatively compare the development of the primary cilium and its length, images of each cell type (Cx43+/+ SCR, Cx43+/+ RNAi α2 and Cx43-/-) were analyzed. These images were taken from different fields using fluorescence microscopy (40X).
Statistical analyzes were carried out comparing the means ± SD of the length in μm of all the cilia analyzed in the cells Cx43+/+ SCR (2.37), Cx43+/+ RNAi α2 (2.08) and Cx43-/- (1.52). The results obtained indicate that the length of the primary cilium in cells in which α2 integrin was silenced and in cells deficient in Cx43 was significantly less than in scrambled cells. The p-value was: p = 0.0017 for the comparison of SCR vs RNAi α2, p < 0.0001 SCR vs Cx43-/- and p < 0.0001 RNAi α2 vs Cx43-/- (Fig. 3).
Based on these observations, we can suggest that connexin 43 and integrin α2 are involved in primary ciliary elongation in osteocytic cells.
REGULATION OF ERK KINASE 1/2 PHOSPHORYLATION BY CONNEXIN 43 IN OSTEOCYTIC CELLS
To characterize the response of MLO-Y4 cells (Cx43+/+ and Cx43-/-) to stimulation by agonists, the PTH1R, PTH and PTHrP (1-37) receptor ligands were added to the culture medium at a concentration of 10-7 M, for 10 min. Immediately afterwards, protein extraction was performed to analyze P-ERK expression by Western blot. The purpose of this experiment was to determine if Cx43 deficiency influenced the activation of the PTH1R receptor after stimulating it with their respective ligands (PTH and PTHrP) and to analyze the intracellular signaling pathways that this stimulus activates and the cellular response that it triggers, specifically the ERK 1/2 phosphorylation.
The results obtained show that in Cx43+/+ cells, stimulation with PTH and PTHrP increases ERK 1/2 phosphorylation (mean ± SD of the duplicates made) with respect to cells that were not stimulated with any ligand (CE). In the case of Cx43-/- cells, PTH stimulation decreased ERK phosphorylation relative to Cx43+/+ cells that were stimulated with PTH, and the agonist peptide PTHrP did not stimulate Cx43-/- cells. No significant variations were obtained in the levels of P-ERK between the different experimental conditions (Fig. 4).

Figure 4. Analysis of P-ERK after stimulating MLO-Y4 Cx43+/+ and Cx43-/- cells with PTH and PTHrP. In Cx43+/+ cells, stimulation with PTH and PTHrP increases ERK 1/2 phosphorylation vs. cells that were not stimulated with any ligand (CE) (2.200 ± 0.696 vs. 0.4240 ± 0.150 and 1.11 ± 0.554 vs. to 0.4240 ± 0.150, respectively). In Cx43-/- cells, PTH stimulation decreased ERK phosphorylation relative to Cx43+/+ cells that were stimulated with PTH (0.865 ± 0.003 vs. 2.20 ± 0.696). Total ERK and tubulin values were used to normalize P-ERK values. Results are expressed as mean ± SD of a duplicate experiment of each experimental condition vs. CE.
Our observations suggest that connexin 43 regulates ligand-activated PTH1R-dependent phosphorylation of ERK 1/2.
CX43 EXPRESSION REGULATES VIABILITY AND ADHESION OF OSTEOCYTIC CELLS
A cell death assay was performed with Cx43+/+ SCR, RNAi α2 and Cx43-/- cells in order to determine whether the lack of expression of integrin α2 and Cx43 would modify the signaling pathways involved in the cell response, causing its apoptosis. Figure 5A shows how the number of live Cx43-/- cells decreases significantly with respect to Cx43+/+ SCR cells (p = 0.0035). In the case of the analysis of dead cells, we see that their number increases significantly in Cx43-/- cells compared to Cx43+/+ SCR (p = 0.0074).
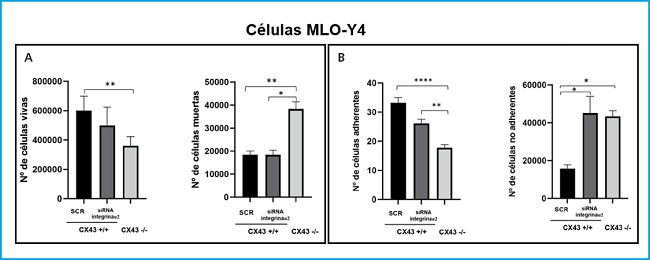
Figure 5. Analysis of cell death (A) and cell adhesion (B) in Cx43+/+ (SCR and α2 ARNi) and Cx43-/- cells. The results show the mean ± SD values of live and dead cell counts from a single experiment with six replicates for each condition, and the counts of adhered and non-adhered cells on the plate. *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001.
As for the Cx43+/+ RNAi α2 cell line, as there were no significant differences in the number of live cells (although there was a slight tendency towards a decrease in their number) and it presented a very similar number of dead cells with respect to cells. Cx43+/+ SCR, it is suggested that the silencing of the α2 integrin influences the proliferation of osteocytes and not their death.
On the other hand, an adhesion assay was also performed on Cx43+/+ SCR, RNAi α2 and Cx43-/- cells. Figure 5B shows that the number of Cx43-/- cells adhered to the collagen plaque decreases significantly with respect to Cx43+/+ SCR and α2 RNAi cells (p < 0.001 and p = 0.0039, respectively). In the case of counting non-adherent cells, their number increased significantly both in Cx43+/+ RNAi α2 cells and in Cx43-/- compared to Cx43+/+ SCR (p = 0.0146 and p = 0.0134, respectively).
Cell adhesion was quantified using brightfield images, which also allowed the morphology of the different cell types to be analyzed, and it was observed that the Cx43+/+ SCR cells had a more elongated shape and a greater number of dendritic extensions, which would potentially allow them to have a higher adhesion capacity. In contrast, Cx43+/+ RNAi α2 and Cx43-/- cells showed a more rounded structure, probably due to the absence of both integrins and connexins, both transmembrane proteins that favor cell adhesion (4).
These results are related to those obtained in the cell death assay, since we observed a greater number of dead cells in the Cx43-/-, due to the lack of Cx43, adhesion to the culture surface would be difficult, which could cause cells to detach and die due to lack of substrate binding.
DISCUSSION
Integrins and connexins play an essential role in cellular functions (14). However, it has not been clarified whether the interaction of both families of proteins is necessary for the proper functioning of osteocytes. The decrease in α2 integrin expression in the Cx43-/- cells led us to consider whether this integrin could act as a mediator in the processes dependent on Cx43 in the osteocytes.
In addition to Cxs and integrins, the primary cilium is also considered a mechanosensor of osteocytes (4). In the immunofluorescence images, it was possible to observe how the deficiency in Cx43 and the silencing of the integrin α2 influence the length of the primary cilium. Various studies indicate that the length of the primary cilium is a determining factor for its correct action as a mechanosensory (4), given that if the length of the cilium is very small, the space of the ciliary membrane would decrease where ion channels and receptors such as PTH1R that mediate the ciliary membrane detected by the cilium and allow the transduction of different intracellular signals (15). Furthermore, the primary cilium generates a specific intracellular compartment in which the intra-flagellar transport of different proteins is necessary both for the development of this organelle and for signal transduction (16). Therefore, if this space is reduced, these processes could be altered, causing the osteocytes not to generate adequate responses to extracellular stimuli (10).
In the immunofluorescence images, a different distribution of Cx43 between Cx43+/+ SCR and Cx43+/+ RNAi α2 cells also seems to be observed. Although in both conditions the expression levels of Cx43 are similar, that is, the silencing of the integrin α2 did not influence the expression of Cx43, it could influence the cellular localization of this protein, since in the Cx43+/+ cells the Cx43 It was found mainly in the cell membrane. These results could indicate that integrin α2 and Cx43 interact in the cell membrane and that their expression triggers a positive feedback favoring the transport of these proteins from the endoplasmic reticulum to the cell membrane.
The osteocytes, embedded in the mineralized matrix, are capable of detecting different signals, translating them into biological responses and transmitting them to the rest of the bone cells, mainly osteoblasts and osteoclasts, to allow the maintenance of bone homeostasis (17). One of these signals is stimulation by PTH1R agonists (PTHrP and PTH) (18).
Our results indicate that ERK 1/2 phosphorylation increases in Cx43+/+ cells when they are stimulated with PTH and PTHrP. In the case of Cx43-/- cells, stimulation with PTH and PTHrP seems to be inhibited since the levels of phospho-ERK 1/2 decrease with respect to Cx43+/+ cells. This may be due to the fact that Cx43-/- osteocytes are less sensitive to stimuli, given that they are deficient in the Cx43 protein that acts as a mechanosensory and also have a shorter primary cilium. All this would cause the PTH1R to have less space to locate itself in the ciliary membrane and generate intracellular responses such as the phosphorylation of ERK 1/2. Another of the results observed in this experiment is that it seems that the increase in ERK 1/2 phosphorylation is greater when we stimulate with PTH than with PTHrP, at the time studied (10 min), which would indicate that both agonists activate PTH1R differently. This activation mechanism is probably mediated by Cx43. However, more experiments would be required to confirm that this stimulation can generate a modification in ERK phosphorylation and therefore a biological response in the cell.
Other characteristics analyzed in these cells were their morphology, adhesion capacity and cell death. The Cx43+/+ SCR cells were found to have a more elongated shape and a greater number of dendritic extensions, which increased their adhesion capacity. In contrast, Cx43+/+ RNAiα2 and Cx43-/- cells have a more rounded morphology and a lower adhesion capacity compared to Cx43+/+ SCR cells. Cx43-/- cells also show a higher mortality compared to the other two cell types. Based on these results, it is suggested that the absence of Cx43 and integrin α2 prevent the correct adhesion of cells to the collagenized plate, which in turn would compromise the development of dendritic extensions (19). Reducing the number of dendritic extensions would cause the communication between cells to be altered and since this communication is essential for cell survival, it could explain why a higher mortality is observed in Cx43-/- cells. The results obtained concur with previous research in which it has been shown that connexins and integrins are proteins that intervene in cell adhesion (20). In addition, Cx43 regulates different signaling pathways that lead to the expression of pro-survival genes21, which is consistent with the results observed. In the case of Cx43+/+ RNAi α2 cells, alterations in cell adhesiveness could cause less pronounced cellular effects, since no alterations in cell survival were observed under these conditions. However, the decrease in adhesiveness could explain the tendency of these cells to proliferate less than Cx43+/+ SCR.
CONCLUSION
Our study indicates that Cx43 deficiency modifies the expression pattern of integrins in MLO-Y4 osteocytes, inhibiting the expression of integrin α2 and increasing that of integrins α6, β1 and β3, which seems to modify their adhesion capacity. In addition, the absence of integrin α2 and Cx43 alters the length of the primary cilium.
Acknowledgments:
we are grateful for the support of the Spanish Society of Bone and Mineral Metabolism (SEIOMM) and the Spanish Foundation for Bone and Mineral Metabolism Research (FEIOMM) that allowed us to prepare this manuscript.
REFERENCES
1. Scheuren A, Wehrle E, Flohr F. Bone mechanobiology in mice: toward single-cell in vivo mechanomics. Biomech Model Mechanobiol 2017;16(1):2017-34. DOI: 10.1007/s10237-017-0935-1 [ Links ]
2. Claude J. Membrane channels formed by gap junction proteins. Biochim Biophys Acta Biomembr 2018;1860(1):1-4. DOI: 10.1016/j.bbamem.2017.10.021 [ Links ]
3. Rupp M. Osteocytes. Z Orthop Unfall 2019;157(02):154-63. DOI: 10.1055/a-0658-5922 [ Links ]
4. Geoghegan IP, Hoey DA, McNamara LM. Integrins in Osteocyte Biology and Mechanotransduction. Curr Osteoporos Rep 2019;17(4):195-206. DOI: 10.1007/s11914-019-00520-2 [ Links ]
5. Plotkin LI, Davis HM, Cisterna BA, Sáez JC. Connexins and Pannexins in Bone and Skeletal Muscle. Curr Osteoporos Rep 2017;15(4):326-34. DOI: 10.1007/s11914-017-0374-z [ Links ]
6. Esbrit P, Alcaraz MJ. Current perspectives on parathyroid hormone (PTH) and PTH-related protein (PTHrP) as bone anabolic therapies. Biochem Pharmacol 2013;85(1):1417-23. DOI: 10.1016/j.bcp.2013.03.002 [ Links ]
7. Ardura JA, Portal-Núñez S, Alonso V, Bravo B, Gortázar AR. Handling parathormone receptor type 1 in skeletal diseases: Realities and expectations of abaloparatide. Endocrinol Metab 2019;30(10):756-66. DOI: 10.1016/j.tem.2019.07.014 [ Links ]
8. Hoey DA, Downs ME, Jacobs CR. The mechanics of the primary cilium: an intricate structure with complex function. J Biomech 2012;45(1):17-26. DOI: 10.1016/j.jbiomech.2011.08.008 [ Links ]
9. Mirvis M, Stearns T, James W. Cilium structure, assembly, and disassembly regulated by the cytoskeleton. J Biochem 2018;475(14):2329-53. DOI: 10.1042/BCJ20170453 [ Links ]
10. Pala R, Alomari N, Nauli SM. Primary Cilium-Dependent Signaling Mechanisms. Int J Mol Sci 2017;18(11):2272-345. DOI: 10.3390/ijms18112272 [ Links ]
11. Batra N, Burra S, Siller-Jackson AJ, Gu S, Xia X, Weber GF, et al. Mechanical stress-activated integrin α5β1 induces opening of connexin 43 hemichannels. PNAS 2012;109(9):3359-64. DOI: 10.1073/pnas.1115967109 [ Links ]
12. Plotkin LI, Mathov I, Aguirre JI. Mechanical stimulation prevents osteocyte apoptosis: requirement of integrins, Src kinases and ERKs. Am J Physiol Cell Physiol 2005;289(3):633-43. DOI: 10.1152/ajpcell.00278.2004 [ Links ]
13. Kato Y, Windle JJ, Koop BA, Mundy GR, Bonewald LF. Establishment of an Osteocyte- like Cell Line, MLO-Y4. J Bone Miner Res 1997;12(12):2014-23. DOI: 10.1359/jbmr.1997.12.12.2014 [ Links ]
14. Plotkin LI, Speacht TL, Donahue HJ. Cx43 and mechanotransduction in bone. Curr Osteoporos Rep 2015;13(2):67-72. DOI: 10.1007/s11914-015-0255-2 [ Links ]
15. Martín-Guerrero E, Tirado-Cabrera I, Buendía I, Alonso V, Gortázar AR, Ardura JA. Primary cilia mediate parathyroid hormone receptor type 1 osteogenic actions in osteocytes and osteoblasts via Gli activation. J Cell Physiol 2020;235(10):7356-69. DOI: 10.1002/jcp.29636 [ Links ]
16. Zhang J, Chandrasekaran G, Li W, Kim DY, Jeong IY, Lee SH, et al. Wnt-PLC-IP3-Connexin-Ca2+ axis maintains ependymal motile cilia in zebrafish spinal cord. Nat Commun 2020;11(1):1860. DOI: 10.1038/s41467-020-15248-2 [ Links ]
17. Cresswell EN, Nguyen TM, Horsfield MW, Alepuz AJ, Metzger TA, Niebur GL, et al. Mechanically induced bone formation is not sensitive to local osteocyte density in rat vertebral cancellous bone. J Orthop Res 2018;36(2):672-81. DOI: 10.1002/jor.23606 [ Links ]
18. Tirado-Cabrera I, Martin-Guerrero E, Heredero-Jimenez S, Ardura JA, Gortázar AR. PTH1R traslocation to primary cilia in mechanically-stimulated osteocytes prevents osteoclast formation via regulation of CXCL5 and IL-6 secretion. J Cell Physiol 2022;237(10):3927-43. DOI: 10.1002/jcp.30849 [ Links ]
19. Rolvien T, Amling M. Disuse Osteoporosis: Clinical and Mechanistic Insights. Calcif Tissue Int 2022;110(5):592-604. DOI: 10.1007/s00223-021-00836-1 [ Links ]
20. Cheng B, Zhao S, Luo J, Sprague E, Bonewald LF, Jiang JX. Expression of Functional Gap Junctions and Regulation by Fluid Flow in Osteocyte-Like MLO-Y4 Cells. J Bone Miner Res 2001;16(2):249-59. DOI: 10.1359/jbmr.2001.16.2.249 [ Links ]
21. Gortazar AR, Martin-Millan M, Bravo B, Plotkin LI, Bellido T. Crosstalk between caveolin-1/extracellular signal-regulated kinase (ERK) and β-catenin survival pathways in osteocyte mechanotransduction. J Biol Chem 2013;288(12):8168-75. DOI: 10.1074/jbc.M112.437921 [ Links ]
Received: July 18, 2022; Accepted: October 13, 2022











 text in
text in 


