INTRODUCTION
The need for regulation of the safety of cosmetic products should be the primary goal. The possibility that these safety issues are recognized makes it a global problem; although these cases are not as common as other types of products such as food, pharmaceuticals or medical devices, there is a need to investigate the mechanisms of implementation of cosmetovigilance system in order to ensure that the cosmetics used by consumers are safe.
What is the Peruvian legislation on cosmetics?
The Law N° 26842 - Health General Law1 states that in Titles I, II and II as follows:
I. Health is a prerequisite for human development and critical to achieving individual and collective welfare environment.
II. The protection of health is of public interest. It is therefore the responsibility of the State to regulate, monitor it and promote it.
III. Everyone has the right to health protection under the terms and conditions established by law.
Additionally, the Law N° 29459 - Law of Pharmaceutical Products, Medical Devices and Health Products establishes the requirements to apply for the Health Authorization. For pharmaceutical products in Peru there is a national pharmacovigilance system that allows health professionals to report adverse reactions. Also, Peruvian Regulatory Agency (DIGEMID) receives reports of adverse reactions by use of medical devices. The Law N° 29459 has two specific regulations: Regulation of Pharmaceutical Facilities (Norm N° 014) and Regulation for registration, control and surveillance of Pharmaceutical Products, Medical Devices and Health Products (Norm N° 016). Although these documents have been approved, the specific guidelines to regulate and support in detail the access and use of pharmaceutical products, medical devices and health products are approved yet, which should have been approved in 2012.
Also, the certifications of Good Pharmacy Practice for community pharmacies express as mandatory the dispensing process for pharmaceuticals products, medical devices and health products (cosmetics are included). Also, it is mandatory as well comply with Good Pharmacovigilance Practice, which implies the reporting of adverse reactions.
On an international level, there are Andean regulations that facilitate the entry of cosmetic products among its member countries (Bolivia, Colombia, Ecuador, Peru and Venezuela). These rules are governed by the following basic principles:
Uniform platform among Member Countries of the Andean Community, to ensure that the right to trade in the products falling within its scope is exercised in a fair and transparent manner;
Balance between safeguarding public health and the free movement of products in the Andean sub region;
Compulsory Sanitary Notification and recognition as market access mechanism, instead of Sanitary Registration;
subsequent control, which allows effectively verify the sanitary quality of the products on the market; and simplification of administrative procedures to facilitate free trade in products between Member Countries of the Andean Community, without undermining the health quality of them.
These actions are part of different documents like the Declaration of Cartagena 2014 Business Council2. It is expected that the Pacific Alliance generates more commercial dynamics, expecting that cosmetic category one of the more impact3 expected, even for the attempt to negotiate as a block with Asian countries4. In this way, Peru needs to ensure safety of new cosmetics which will arrive to Peruvian market.
Aim of the study
Determinate what the barriers in Peruvian community pharmacies for cosmetovigilance practice are?
Research model
For the current study, it was designed a research model to be evaluated. Figure 1 shows the research model.
METHODS
Sample selection
It was established obtain the information from pharmacists working in community pharmacies in 2 districts in Lima, Peru. 81 self-administered anonymous questionnaires were delivered and returned by the research team.
Questionnaire development
For the current study, it was built a questionnaire ad hoc. The instrument comprised two sections. The first section captured demographic information as sex, age, education level, year of experience and their position in the pharmacy. The second part included a 5-point Likert scale, where 1=strongly agree and 5=strongly disagree. It was tested 16 items, distributed in 4 dimensions: resources, interest, regulation and pharmacist practice. The evaluation the reliability, it was used SPSS software V.24, and it was calculated the Cronbach's of 20 completed questionnaires because for internal consistency reliability, the composite reliability is considered adequate when an item o dimension has a factor loading greater than 0.707 as expressed by Carmines & Zeller5. Cronbach's values of each dimension were 0.711, 0.800, 0.709 and 0.865, so it did not need changes in items of questionnaire.
Validation with SEM PLS
Using Partial Least Squares Structural Equation Modeling (PLS-SEM) was analyzed the validity of construct and discriminant and, internal consistency by the composite reliability. SmartPLS statistical package6 was used to calculate the factorial structure of the indicators, using Partial Least Squares. SEM-PLS aims to predict the latent variables by estimating Partial Least Squares (PLS) and Principal Component Analysis (PCA). The main advantage of PLS is the greatest strength calculations to smaller samples and breach of statistical assumptions of the variables (non-normal distribution, different levels of measurement, multicollinearity, among others). In a PLS model, the individual reliability of the indicators is assessed by examining the load between each indicator and dimension, accepting as reliable those above 0.40 loads for exploratory studies7.
Informed consent was obtained from each participant of current study, following in accordance with The Code of Ethics of the World Medical Association.
RESULTS
Out of 81 questionnaires of distributed in 2 districts in Lima were fulfilled (response rate = 51.9%). Regarding the pharmacists who answered the survey, 72.83% were women. The average age was 35.72 years (SD: 5.68). Average of years of working in community pharmacies was more than 6.36 years (SD: 4.63). Table 1 presents the analysis of factor including the Cronbach's for each variable. Cronbach's values were more than 0.707, which is considered statistically acceptable.
Table 1 Analysis of factors
| Items | Factor | Cronbach's Alpha |
|---|---|---|
| Resources | 0.708 | |
| Lack of time | 0.805 | |
| Lack of space in the pharmacy | 0.786 | |
| Lack of knowledge about cosmetovigilance | 0.595 | |
| Interest | 0.804 | |
| My interest to report adverse reactions by cosmetics | 0.795 | |
| Interest of managers/owners to report adverse events by cosmetics | 0.717 | |
| Interest of customers to report adverse events by cosmetics | 0.757 | |
| Interest of regulators to create a system to report adverse events by cosmetics | 0.743 | |
| Interest of third parties to create a system to report adverse events by cosmetics | 0.713 | |
| Regulation | 0.701 | |
| Lack of specific regulation about cosmetovigilance | 0.610 | |
| Lack of health authority promotion of reports of adverse events by cosmetics | 0.657 | |
| National health care structure in general | 0.870 | |
| Lack of clinical practice guideline focus in cosmetovigilance | 0.743 | |
| Cosmetovigilance practice | 0.865 | |
| Rarely I ask to customers about adverse reaction with cosmetics use | 0.691 | |
| Rarely I explain to patients/customers about adverse reaction with cosmetics use | 0.897 | |
| Rarely I suggest to customers to report adverse reaction with cosmetics use | 0.878 | |
| Rarely I have education material about adverse reaction with cosmetics use | 0.901 |
More than 70% of the participants chose the «strongly agree» and «agree» alternative for items about barriers that included:
Also, about daily activities, more than 70% of the participants chose the «strongly agree» and «agree» alternative for items that included:
Rarely I explain to patients/customers about adverse reaction with cosmetics use
Rarely I have material to inform to customers about adverse reaction with cosmetics use
Evaluation of hypotheses
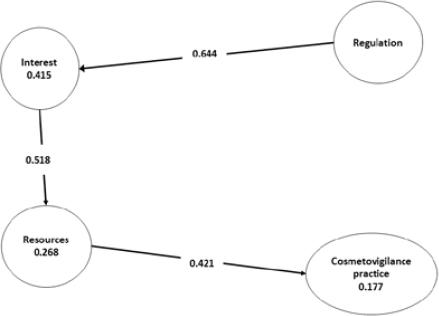
Considering the relation showed in figure 2, we evaluated the hypotheses proposed.
H1. Regulation barriers has a positive influence on Interest barriers
Regulation barriers have a positive influence over Interest barriers observing that the path coefficient is 0.644 for the Regulation endogenous latent variable. Also, data shows that the Regulation barriers explain 41.5% of the variance of Interest barriers.
H2. Interest barriers has a positive influence on Resources barriers
Interest barriers have a positive influence over Resources barriers observing that the path coefficient is 0.518 for the Interest endogenous latent variable. Also, data shows that the Interest barriers explain 26.8% of the variance in Resources barriers.
H3. Resources barriers has a positive influence on Cosmetovigilance Practice
Resource barriers have a positive influence over Cosmetovigilance Practice observing that the path coefficient is 0.421 for the Resources endogenous latent variable. Also, data shows that the Resources barriers explain 17.7% of the variance in Cosmetovigilance Practice.
The composite reliability for each latent variable was:
Resources: 0.707 Interest: 0.862
Regulation: 0.815 Cosmetovigilance Practice: 0.909
DISCUSSION
About resources, lack of time is usually a problem for care patients in community pharmacies which perform activities focus basically in sells products instead of care patients. Also, many customers arrive to community pharmacies so it is not feasible can take time with one of them and have the rest of customers waiting while pharmacists explain about use or safety issue.
Specific space in pharmacies is an expected barrier because customers need a private area to talk about their personal problems with use of cosmetics which can be light problems until serious events. Knowledge about cosmetic is essential to recognize adverse events with use of cosmetics by consumers but there are not available specific education in universities undergraduate programs for detect adverse events by cosmetics which explain low level of training in community pharmacists about this issue.
An important item reported was «my interest to report adverse events by cosmetics». This item can show a deep problem because the pharmacists express their lack of interest about report adverse events. It is can be explained by lack of previous models, lack of training previous and lack of safety goals in community pharmacies.
Managers and owners of community pharmacies, mostly no pharmacists, cannot find any benefits of get and report adverse events by cosmetics to regulatory agency. Commercial focus of this group explain lack of interest; also, pharmacists express that the customers are not interest; however, Di Giovanni et al.8 showed that consumers reported adverse cosmetic events whereby future studies are need to know perspectives of customers. Both regulators and third parties don't have cosmetovigilance as a tool to improve safety in patients and even reduce costs of health due to damage of patients.
Regulation is the main gap of lack of cosmetovigilance practice. As it was explained above, the elaboration and publication of norms that promote the cosmetovigilance in Peru are pending. Also, independent of national regulation, guidelines can be developed to be used in daily activities in community pharmacies but again, this kind of document is not available yet. Cosmetics Europe Guidelines on the Management of Undesirable Effects and Reporting of Serious Undesirable Effects from Cosmetics in the European Union9 can be used as a guide for implementation of cosmetovigilance in practice as previously have described by Zakaria10 about Malaysia health system. Also, other authors have reported need of cosmetovigilance as Udupa y Ligade11 in India.
Finally, pharmacists reported that they rarely develop activities focus in cosmetovigilance practice to get information from customers about adverse cosmetic reaction. Daily activities of community pharmacists show the impact of current barriers to not allow the implementation of cosmetovigilance.
CONCLUSION
Cosmetics legislation does not include any element which is required cosmetovigilance since trade agreements with other economic blocks will Peruvian citizens at greater risk in using different products. The absence of specific legislation is a major constraint but also it is the lack of professionals with experience in this kind of specific regulation for cosmetics. The virtualized start notifications will ensure a systematization of notifications, having to take into account different means according to the ages and behavioral preferences of citizens. The pharmacist's views expressed barriers as resources, interests and regulation. Also, they expressed that their daily activities are not focus in reports of customers of adverse cosmetic events. The need the cosmetovigilance is issue of public health to take responsibility for care customers against cosmetic. The implementation of cosmetovigilance, regardless of the institution responsible for the regulation of cosmetics, is an urgent task. Consumers need it.