INTRODUCTION
Licorice or Liquorice is the dried peeled or unpeeled roots and stolons of Glycyrrhiza glabra Linn, Family: Fabaceae . This plant has been used for its medicinal property for more than 4000 years.
Licorice has proved to be effective in the treatment of disease like gastric ulcers, arthritis, allergy, inflammation, leukemia, cancer, psoriasis, atopic dermatitis and in hepatotoxicity 1 . Basically, licorice comprises of two components i.e. glycone and aglycone which are responsible for its medicinal properties. Glycone is glycyrrhizic acid (GA) and aglycone is glycyrrhetinic acid, Out of these, glycone part i.e. GA is an important compound responsible for the pharmacological and biological properties of licorice. GA is a triterpenoid, diasaccharide glycoside proved to attain anti-inflammatory property, anti-diabetic, anti allergic and many more properties 2, 3, 4.
GA exhibit the mechanism of curing inflammation by inhibiting the generation of reactive oxygen species produced by neutrophils 5, 6.
GA has molecular weight of 822.93 g/mol and molecular formula C 42H62O16, having a physical appearance as a yellow to orange color powder. The objective of the present investigation was to extract GA from licorice roots.The extracted GA was validated by physicochemical parameters, thin layer chromatographic analysis (High-performance liquid chromatography). In vitro technique was opted for evaluating its anti-inflammatory property 7, 8, 9, 10.
METHODOLOGY
Physicochemical analysis
Total ash: Total ash was calculated by incinerating the fine powder of crude drug (2g) in a tarred silica crucible at the temperature of 450ºC such that there is complete removal of carbon. After that the ash obtained was allowed to cooled and weighed. The percentage of total ash was calculated using the weighed value of ash and powdered crude drug.
Acid insoluble ash: The ash value was determined for detecting the undesirable or harmful or earthy matter which can be present in the crude drug. For estimating acid insoluble value, the ash obtained from the above method was poured in 25 ml of dil. HCl kept on heating mantle. The mixture was filtered using the ash less filter paper, washed with hot water, ignited and weighed.
Water soluble ash: For determining the water soluble ash value the ash obtained from the total ash procedure was used and mixed with 25 ml of water. The mixture was filtered and the mixture obtained on the filter paper was collected and weighed. This weighed quantity of insoluble matter was subtracted from the weighed of ash for obtaining water soluble ash value. This weighed quantity was used for calculating the percentage of water soluble ash value.
Determination of Extractive values
Alcohol and water extractive values were determined using the same procedure except the use of alcohol to determine alcohol extractive value and the use of water in case of calculating water soluble extractive value. To determine the extractive value, powdered crude drug was macerated with the respective solution (alcohol for alcohol extractive value and water for water extractive value) in a closed flask for 24h (shaking frequently for the starting six hours). The solution was filtered and 25 ml of the filtrate was evaporated to dryness in tarred petri plate. The solution was kept at 105ºC and the finally the rest amount was weighed. 9 , 11
Extraction of glycyrrhizic acid from licorice root
GA was extracted by using the method of maceration with slight modification in the method described in literature. For this purpose, drug (licorice roots) powders was macerated with the solvent mixture of acetone and dilute nitric acid for 2 h. The contents were filtered and additional 20 ml of acetone was added to the marc and warmed gently. The contents were filtered and filtrate was obtained. To this filtrate sufficient volume of dilute ammonia solution was added till precipitation of ammonium glycyrrhizinate is completed. The precipitate was collected and washed with 5 ml of acetone, dried and collected 12 .
Phytochemical screening of GA
Phytochemical screening of GA was performed for the identification of phytoconsituents present.
Test for saponins (foam test)
The extract was dissolved with 20 ml of distilled water and stirred for 15 minutes. The formation of 1cm layer of foam for a period of time showed the presence of saponins.
Tests for flavonoids
With sodium hydroxide: Extract was mixed with 1 ml of sodium hydroxide solution. Blue to violet color indicates the presence of anthocyanins, yellow to orange color shows the presence of flavonones and yellow color indicates flavones.
With concentrated sulphuric acid: Extract was mixed with concentrated sulphuric acid. Yellow orange color indicates the presence of anthocyanins, orange to red color indicates the presence of flavones.
Shinoda test: For performing shinoda’s test, extract was dissolved in ethanol, to which magnesium turnings were added. To this mixture, Conc. Hydrochloric acid was added. Turning of magenta to purple color indicates the presence of flavonoids.
Test for terpinoids
Lieberman’s test: Acetic acid was added to the extract kept on the hot plate. To this mixture, concentrated sulphuric acid was added. Presence of pink color indicates the presence of triterpinoids in the extract.
Trichloroacetic acid test: tricholoroacetic aid was added to the extract. Formation of yellow color indicates the presence of terpinoids.
Fehling’s test
On the water bath, Extract was kept. To which Fehling solution A and B were mixed. Brick red precipitate showed the presence of reducing sugars. 9 , 13 , 14
Loss on drying
Loss on drying was calculating by the mentioned procedure. Weighed quantity of extract was poured onto a weighed petri plate. The petri plate was kept in oven and weighed at different time interval at 105ºC, till two consecutive weighing didn’t differ by more than 0.25mg which indicates the final loss of moisture present in the drug. Percentage loss on drying was calculated using the below mention formula 9 , 15 , 16 .
LOD(%) = weight of porcelain dish with drug at time 0 – weight of porcelain dish after 6 h ÷ weight of porcelain dish at time 0 – weight of empty porcelain dish
pH determination
The extract was dissolve in 10 ml of distilled water for evaluating the pH. The pH was determined using digital pH meter. The pH was measured in triplicate.
Melting point range
The melting point was determined by capillary technique. The drug was filled in the capillary tube, sealed to the one end. The capillary was introduced into digital melting point apparatus. The temperature, at which the drug melts, signifies the melting point of drug.
UV spectral analysis
A UV spectrum of the extracted GA was obtained by scanning the extract solution in the range of 200-800nm using UV spectrophotometer. For this, stock solution of 100µg/ml of GA solution was prepared 17 .
Analysis using Thin Layer Chromatography (TLC)
For TLC test solution, standard solution and developing solvent system were prepared. For preparing test solution, alcohol and water (7:3) was mixed to licorice extract. The solution was heated using water bath for 5 minutes, cooled and filtered. Standard solution was prepared by dissolving 5 gm of standard glycyrrhizic acid in 1ml of a mixture of alcohol and water (7:3). Developing solvent system contains the mixture of butyl alcohol, water and glacial acetic acid (7:2:1). TLC plates were prepared using silica gel solution and the retention value (Rf.) was calculated. The plates were kept in developing solvent system and examined under UV light at 254nm 18 .
In vitro anti inflammatory activity
The In Vitro anti inflammatory activity of GA was evaluated by albumin denaturation technique as given by Mizushima and kobayashi with slight modification. Extract was mixed with 1% aqueous solution of fetal bovine albumin. pH of the mixture was adjusted using 0.1NHcl. The solution was kept in a incubator at 37ºC for 20 min 19 , 20 , 21 . Afterwards, denaturation was induced by keeping the reaction mixture at 60±1°C in water bath for 10 min. The mixture was cooled and the turbidity was measured using UV spectrophotometer. Percentage inhibition was calculated using the following equation. For this, acetyl salicylic acid was considered as a standard and the solution containing no drug was considered as control 22 , 23 , 24 .
% Inhibition = (Absorbance of control – Absorbance of sample) × 100 / Absorbance of control
RESULTS
Physicochemical analysis
Physicochemical parameters like ash value, acid insoluble, water soluble and alcohol soluble value were carried out. The results showed that all the values obtained were in the limit as given in Ayurvedic Pharmacopoeia of India (API). The total ash value was found to be 3.75%. The values of acid insoluble, water soluble extractive and alcohol soluble extractive value were found to be 1.93%, 3.51% and 2.18% respectively as given in Table 1 , indicating the presence of water soluble components than alcohol soluble components.
Table 1. Compiled results for total ash, acid insoluble ash, water soluble extractives and alcohol soluble extractive values as compared to standard values
| S. No. | TESTS | OBSERVATIONS (%) | STANDARD % (API) |
|---|---|---|---|
| 1 | Total ash | 3.75 | Not more than 10 |
| 2 | Acid-insoluble ash | 1.93 | Not more than 2.5 |
| 3 | Water soluble extractives | 3.51 | Not less than 20 |
| 4 | Alcohol soluble extractives | 2.18 | Not less than 10 |
Phytochemical screening of GA
The tests performed for Phytochemical screening showed the presence of flavonoids, saponins and triterpinoids in the extract (GA) as shown in Table 2.
Table 2 Observation found via phytochemical tests
| S. No. | TESTS | OBSERVATIONS | RESULT | |
|---|---|---|---|---|
| 1 | Fehling test | Brick red precipitate | + | |
| 2 | Test for Flavonoids | With sodium hydroxide | Yellow color | + |
| With sulphuric acid | Orange to red color | |||
| Shinoda test | Magenta to purple color | |||
| 3 | Test for Saponins Foam Test | Persistence of foam for a period of time | + | |
| 4 | Test for terpinoids | Lieberman’s test | Pink color | + |
| Trichloroacetic acid test | yello Yellow color w color | |||
NOTE: ‘+’ Indicates the presence of the compound.
The extract was evaluated for the parameters like pH, melting point and loss on drying. The loss on drying after 6 hours was found to be 6.5%. According to the monograph, the extract should not loose not more than 12.0% of its weight. The value for loss on drying has been summarized in Table 3. The pH of the extract was evaluated in triplicate and the average was found to be 5.53 ± 0.05 as depicted in Table 4. The melting point was found to be -240ºC, which is comparable to the reported melting point of GA i.e.-220ºC.
UV spectral analysis
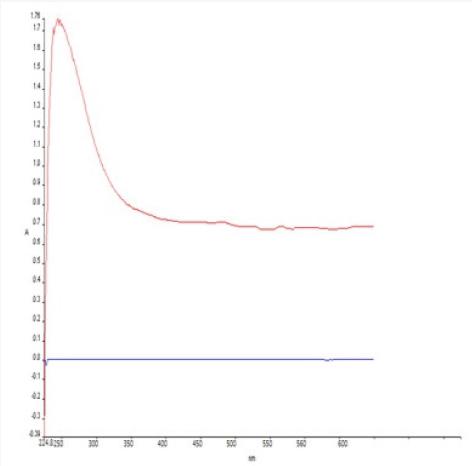
Spectral analysis was evaluated using UV spectrophotometer. The highest peak was attained at the wavelength of 230nm. As reported, the standard wavelength of GA is 254nm. The graph obtained via UV spectrophotometer has been depicted in Figure 1.
Thin layer chromatographic analysis
Rf value was obtained through TLC analysis by dividing the distance travelled by solute from the distance travelled by solvent. The Rf value was found to be 0.5 cm. The TLC plate showed purple color when seen through UV florescent light.
Rf = Distance travelled by solute / Distance travelled by solvent
= 3.5 / 7.0
= 0.5 cm
In vitro Anti-inflammatory activity
In vitro anti inflammatory studies were carried to relate anti inflammatory activity of GA with standard drug, salicylic acid (most commonly used as anti inflammatory agent). Percentage inhibition was calculated using the absorbance and it was found that the extract and standard showed 27.11 % inhibition and 49.15 % inhibition respectively. The results have been compiled in Table 5 .
DISCUSSION
Results obtained from physicochemical analysis (as shown in Table 1 ) showed that there was significant difference found between total ash and acid insoluble ash , which indicates that the ash contains a considerable amount of inorganic radicals like calcium oxalate which are acid soluble. Phytochemical screening revealed the presence of flavoinoids, saponins and triterpinoids in the extract. The in-vitro anti-inflammatory evaluation showed the inhibition of 27.11% from which it can be concluded that the extract has less inflammatory property than standard, but is able to shows a significant percentage of inflammatory property.
CONCLUSION
Glycyrrhizic acid was extracted from licorice roots and evaluated for physiochemical, phytochemical analysis, extractives value. The calculated Rf value of the extract was found to be 0.5 cm. from the results obtained from evaluation parameters it can be concluded that the extracted glycyrrhizic acid contains less and permissible amount of impurities. Glycyrrhizic acid was also evaluated for its anti-inflammatory activity using in vitro analysis, which proved its ability on counteracting on inflammatory activity.