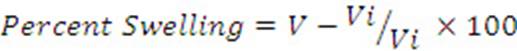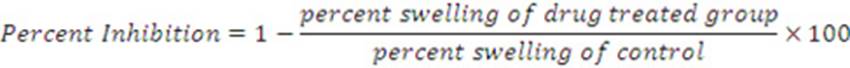INTRODUCTION
Ketoprofen, 2-(3-benzoylphenyl)-propionic acid is a non-steroidal anti-inflammatory agent widely used for the treatment of inflammatory diseases1. In recent decades, the skin has become a well-known administration site for topical and systemic drug delivery. Transdermal delivery has a variety of advantages compared with the oral route. Mainly, it is used when the drug shows significant first-pass effect2. Further, eliminating the problems associated with decreased gastrointestinal absorption. Therefore, transdermal administration is beneficial when a constant drug effect is desired3. It is therefore desirable to develop a nanoformulation system, which does not require the use of penetration enhancers to facilitate drug permeation through the skin. One of the most promising techniques for enhancement of transdermal permeation of drugs is the nanoemulsion techniques4. Nanoemulsion is defined as a dispersion consisting of oil, surfactant, cosurfactant, an aqueous phase, which is a single optically isotropic and thermodynamically stable liquid solution with a droplet diameter usually in the range of 10- 200 nm5. The nanoemulsion has a number of advantages such as to increase drug solubility, thermodynamic stability, and transdermal permeation ability6. The nanosized droplets leading to the enormous interfacial area associated with nanoemulsion would influence the transport properties of the drug, an important factor in sustained and targeted drug delivery7,8. In present work, chitosan is used as a gelling agent for ketoprofen nanoemulsion in order to retain the formulation on the skin for a longer period. Chitosan has been recognized for its variety of application including drug delivery, biomedical, and wound healing acceleration, anti-inflammatory 9,10,11,12. The present study aims to formulate (nanogel) of ketoprofen for better permeation potential through the skin for pain management.
MATERIAL
Ketoprofen was a gift sample from Shreya Life sciences (Aurangabad, Maharashtra, India). Transcutol P, Lauroglycol 90, Peceol, Labrafil M 1944CS, Plurol Oleique CC 497, Labrafac Lipophile WL 1349 were a gift sample from Gattefosse (Mumbai, India). Chitosan (85 % deacetylated) was purchased from Research Lab, Mumbai, India. Tween 80, PEG 400, ethanol, acetic acid and oleic acid were purchased from Merck (Mumbai, India). Deionised water was prepared by Ultra clear TWF UV system (SG Wasseraufbereitung und Regenerierstation GmbH, Germany). All other chemicals used in the study were of analytical reagent grade.
METHODS
Solubility study
The solubility of the ketoprofen was determined in various excipients by taking excess amount of drug in 2 ml of each of the selected excipients in 5 ml capacity stopper vials. The vials were then kept at 25 ± 1.0°C in the orbital isothermal shaker (Lab enterprise, Bombay) for 72 hr to get equilibrium. The equilibrated samples were removed from the shaker and centrifuged at 2000 rpm for 10 min. The supernatants were taken, filtered and analyzed for drug content using UV-Visible spectrophotometer (UV-1700, Pharmaspec, Shimadzu Ltd, Japan) at λmax 260nm13.
Construction of pseudo-ternary phase diagrams
Based on the solubility studies, oleic acid was taken as the oil phase, tween 80 as surfactant and ethanol as a co-surfactant. Distilled water was used as an aqueous phase. Surfactant and co-surfactant (Smix) were mixed at different mass ratios (1:1, 1:2, 1:3). For each phase diagram, oil and Smix were mixed at a specific ratio (1:9 to 9:1) in different vials. Nine different combinations of oil and Smix, 1:9, 2:8, 3:7, 4:6, 5:5, 6:4, 7:3, 8:2, 9:1 at each Smix (1:1, 1:2, 1:3) were made so that maximum ratios were covered for the study (Chemix Ternary Plot software). Slow titration with aqueous phase was performed for each mass ratio of oil and Smix and visual observations were made for transparent and easily flowable o/w nanoemulsions. The region of the nanoemulsion was marked on a pseudo-three-component phase diagram14.
Selection and preparation of ketoprofen loaded nanoemulsion formulation
From the phase diagram constructed, different formulas were selected from the nanoemulsion region so that ketoprofen could be incorporated into the oil phase. The drug was accurately weighted to represent 2.5% of the total weight of the formulation and added to the previous mixture and stirred with a magnetic bar on the magnetic stirrer, at room temperature until the drug completely dissolved. The weighed amount of water then added drop-wise with continuous mixing15.
Thermodynamic stability studies
The formulations selected from pseudo-ternary phase diagrams were further subjected to heating-cooling cycle, centrifugation test, and Freeze-thaw cycle16,17. The formulations, which were stable, are selected for further characterization.
Viscosity
The viscosity of nanoemulsion was determined using Brookfield cone and plate rheometer (RS - CPS + Rheometer, CP75*1) at 25 ± 0.5ºC 15.
Refractive index
The refractive index of placebo and nanoemulsion formulation was determined using an Abbe’s type refractometer at 25 ± 0.5°C (18) (19.
Droplet size and PDI
Droplet size distribution and the polydispersity index of nanoemulsions were determined by using a Delsa Nano-C (Beckman Counter instruments) [18]. The sample of optimized nanoemulsion was suitably diluted with little amount of distilled water.
Transmission electron microscopy (TEM)
Morphology and structure of the nanoemulsion were studied using transmission electron microscopy (TEM) (Philips CM-10, USA) operating at 200kV and capable of point-to-point resolution. The approximately the drop (50μl) of the nanoemulsion was suitably diluted with distilled water (1:100), filtered through 0.45μm filter paper and applied on the carbon-coated copper grid with 1% phosphotungstic acid. It was left for 30 sec for drying purpose. The dried coated grid was placed over the slide and covered with a coverslip for TEM observations.
Preparation of nanogel
The nanoemulsion C1 was selected as the optimized formulations based on results obtained from the above characterization. Further, it was converted into a chitosan nanogel formulation. The chitosan (1% w/w) was used; it was initially dissolved in a solution of acetic acid 0.5% v/w. The 10 ml of optimized C1 formulation was slowly added to the viscous dispersion of chitosan (1% w/w) with continuous stirring on a magnetic stirrer (Remi Mechanical Stirrer, Mumbai, India) to obtain a completely homogenous mixture. The prepared nanogel formulation was stored in a sealed container20.
Ex-vivo permeation study
Excised human cadaver skin (HCS) from the abdomen was obtained from Govt. Medical College, Aurangabad. The skin preparation was done for ex-vivo permeation study 21. Skin penetration studies were carried out by using a modified diffusion cell. The cell had a diffusion surface area of 4.9 cm2 and a volume of 13 ml in the receptor compartment. HCS was mounted horizontally on the receiver compartment. Initially, the donor compartment was empty and the receiver chamber was filled with pH 7.4 phosphate buffered solution. The receptor fluid was constantly stirred with mentioning constant temperature at 37 ± 0.50C. The buffer mixture solution was replaced with fresh buffer every 0.5 h to stabilize the skin. It was found that the receiver fluid showed negligible peak absorption after 5 hr and beyond, indicating complete stabilization of the skin. After complete stabilization of the skin, the 5 ml nanoemulsion and 0.5 gm gel were placed into each donor compartment. Samples were withdrawn at one hour intervals up to 10 h and analyzed for drug content by using UV-Visible spectrophotometer (UV-1700, Pharmaspec, Shimadzu Ltd, Japan) at λmax 260 nm and permeability parameters such as permeability coefficient (Kp) was calculated by dividing the flux (Jss) by initial concentration in the donor compartment and flux was obtain from straight line by plotting the cumulative amount of ketoprofen permeated per unit area of skin versus time at steady state condition 20.
In vivo study
Protocol to perform the anti-inflammatory activity of optimized formulation on the animal was approved by the Institutional animal ethical committee (IAEC) of Dr. VVPF’s College of Pharmacy, Ahmednagar, India (COPH/IAEC/2017/Protocol No.10). The anti-inflammatory activity of ketoprofen loaded nanoemulsion-based gel was evaluated using carrageenan induced hind paw edema method. The healthy male rats, weighing 120-250g, were randomly divided into three groups each containing six rats. The rats were given free access to water and food. The rats were kept under observation for 24 h. The backsides of rats were shaved 12h before starting the experiment. The optimized nanogel formulation for the first group and C1 for second group were applied on the shaved back of all animals (except third control group) half an hour before subplantor injection of carrageenan in the right paw of rats. Paw edema was induced by injecting 0.1 ml of a 1%w/v homogeneous suspension of carrageenan in double distilled water22. The volume of paw edema was measured immediately (0 h) and at 1hr interval up to 5hr after injection using a plethysmometer. The amount of paw edema swelling was determined time-to-time and expressed as percent edema relative to the initial (0 h) hind paw volume. The mean value of the percentage was determined for each time interval. Percentage inhibition of edema produced by each formulation treated group was calculated against the respective control group using the following formula.
The percent swelling of the paw was determined by using the following equation,
Where, V is the paw volume of the carrageenan injection after 8h, and Vi is the initial paw volume. The average paw swelling in the group of the drug-treated rats was compared with control rats and the percent of inhibition of the oedema formation was determined using following equation23,
Stability studies
The stability study was performed accordingly to ICH guideline by storing the optimized nanogel formulation in transparent seal vial at 40±2°C / 75%RH in stability chamber (Lab- Care, Mumbai) for six months. The samples were withdrawn after six months and analyzed for drug content and viscosity24.
RESULTS AND DISCUSSION
Solubility study
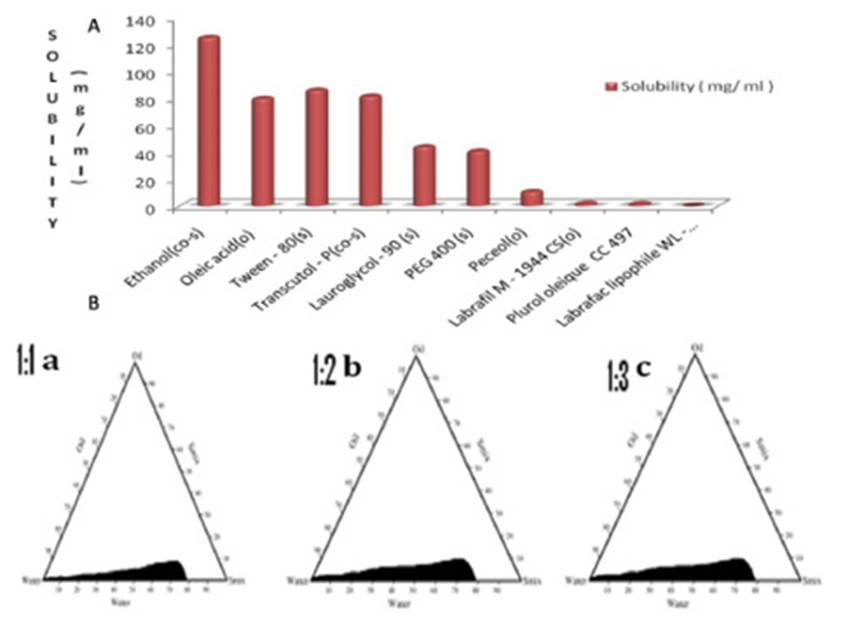
In the solubility study, it was observed that the maximum solubility of ketoprofen was found in oleic acid as compared to the other oils. Moreover, oleic acid also acts as a penetration enhancer for transdermal delivery. Transient negative interfacial tension and fluid interfacial film are rarely achieved by the use of single surfactant; therefore usually, the addition of a co-surfactant is necessary [17]. The co-surfactant selected for the study was ethanol which shows higher drug solubility, also acts as a permeation enhancer and has an HLB value of 4.2. The solubility of ketoprofen in different excipients is shown in Figure 1.
Pseudo-ternary phase diagrams
Pseudo-ternary phase diagrams (PTD) as shown in Figure 1 were prepared separately for each Smix ratio i.e. 1:1, 1:2 and 1:3 so that o/w nanoemulsion region can be identified and optimized for further study. The nanoemulsion region was identified and shaded on PTD for further optimization. Pseudo-ternary phase system with tween80: ethanol (1:3) exhibited a maximum area for nanoemulsion formation. The wider region indicated better nanoemulsifying efficiency of the developed formulation and better interaction among oil phase, Smix, an aqueous phase. In Fig.1a, the Smix ratio 1:1 has a very low nanoemulsion area. The maximum concentration of oil that could be solubilized in the phase diagram was only 7.73% wt/wt using 67.67 % wt/wt of Smix. As the co-surfactant concentration was increased in the Smix ratio 1:2, a slight higher nanoemulsion region was observed, perhaps because of further reduction of the interfacial tension. The maximum concentration of oil that could be solubilized in the phase diagram was 7.40 % wt/wt using 64.73% wt/wt of Smix. As we further increased co-surfactant concentration, Smix 1:3, the nanoemulsion region increased as compared with the region in 1:1 and 1:2. The maximum concentration of oil that could be solubilized by this ratio was 7.2% wt/wt using 63.09% wt/wt of Smix. The formulations which are clear and transparent and easily flowable are selected for further thermodynamic stability study.
Thermodynamic stability of nanoemulsions
The selected formulations were passing the thermodynamic stability tests. Therefore, it revealed that developed formulations are physically and chemically stable. The composition of selected formulations is given in Table 1.
Table 1 Composition of the selected nanoemulsion
| Formulation | Oleic acid (%w/w) | Smix (%w/w) | Water (%w/w) | Ratio of S/Co-s | Ketoprofen (%w/w) |
|---|---|---|---|---|---|
| A1 | 3.49 | 68.51 | 27.98 | 01:01 | 2.5 |
| A2 | 7.73 | 67.67 | 24.57 | 01:01 | 2.5 |
| B1 | 3.28 | 64.28 | 32.43 | 01:02 | 2.5 |
| B2 | 7.40 | 64.73 | 27.86 | 01:02 | 2.5 |
| C1 | 3.09 | 60.54 | 36.36 | 01:03 | 2.5 |
| C2 | 7.2 | 63.09 | 29.69 | 01:03 | 2.5 |
Viscosity
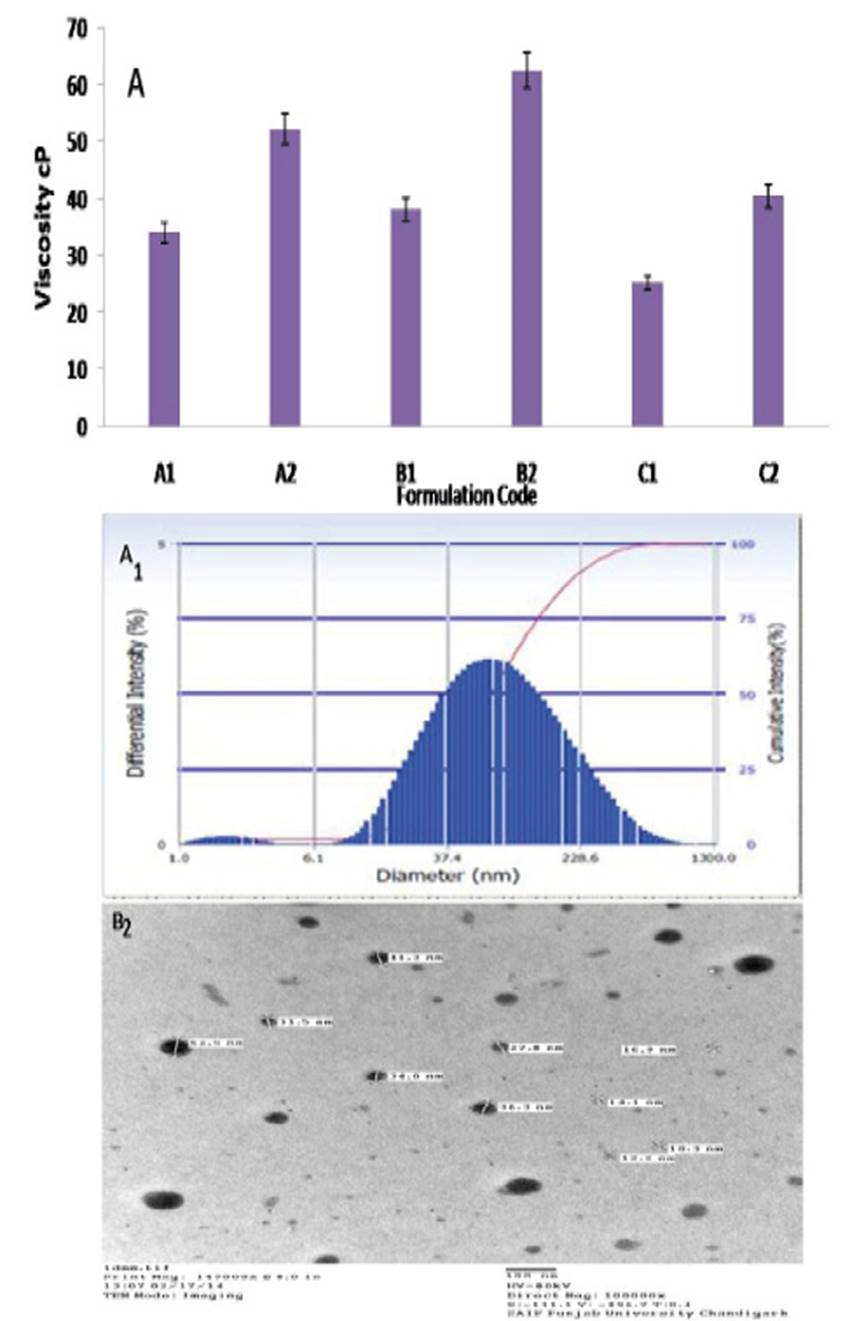
The viscosity study results revealed that viscosity increase with an increase in the oil content shown in Fig.2 A. The formulation A1-A2, the oil concentration was increased from 3.49 % w/w to 7.73 % w/w, and at the same time water concentration decrease from 27.98% w/w to 24.57 % w/w, which leads to the increase in the viscosity from 34.0 ± 2.40 cp to 52.20 ± 3.70 cp. The formulation B1-B2, the oil concentration was increased from 3.28 % w/w to 7.40 % w/w, an increase in the viscosity from 38.10± 4.62 cp to 62.50± 5.45cp was observed. The same observation was obtained in formulation C1-C2. The viscosity of the C1 was found to be very low (25.20± 2.74 cp) as compared to other formulation. This might be due to the presence of low concentration of oil in the formulation and more water content. The same observation was reported that low oil content leads to lower viscosity values 25.
Refractive index (RI)
RI is an optical property of nanoemulsion and which can be used to indicate the isotropic nature. When the refractive index values of all formulation were compared with those of the placebo, it was found that there was no significant difference between the values of RI of water as shown in Table 2. The result revealed that the developed formulation is isotropic in nature and also no interaction between nanoemulsion components.
Table 2 Droplet size, PDI and Zeta potential, viscosity, flux and refractive index of formulations
| Formulation code | Droplet size (nm) | PDI | Zeta potential (mV) | Permeability Coefficient (Kp) | Flux (JSS) (µg/cm2/hr) | Refractive index | |
|---|---|---|---|---|---|---|---|
| Placebo | Formulation | ||||||
| A1 | 63.8 ± 4.04 | 0.355 | -7.55 ± 0.02 | 0.002062 | 24.75± 5.20 | 1. 389 | 1.399 ± 0.02 |
| A2 | 132.0 ± 2.4 | 0.280 | -21.90 ± 2.63 | 0.000920 | 11.05± 3.12 | 1.390 | 1.395 ± 0.01 |
| B1 | 57.2 ± 3.01 | 0.346 | -12.92 ± 0.4 | 0.00248 | 29.77± 4.25 | 1.391 | 1.451 ± 0.02 |
| B2 | 98.3 ± 5.2 | 0.387 | -25.36 ± 1.20 | 0.001157 | 13.89± 2.15 | 1.393 | 1.399 ± 0.02 |
| C1 | 56.5 ± 4.2 | 0.270 | -31.87 ± 0.01 | 0.002972 | 35.67± 3.28 | 1.390 | 1.389 ± 0.01 |
| C2 | 81.7 ± 1.02 | 0.377 | -13.93 ± 0.12 | 0.001560 | 18.72 ± 3.10 | 1.387 | 1.386 ± 0.02 |
| Control | 0.000497 | 5.97 ± 3.10 | |||||
| Nanogel | 0.004170 | 50.12 ± 5.12* | |||||
| Conventional gel | 0.002120 | 25.45± 7.24 | |||||
All values are expressed as mean ± SD, n = 3. * P<0.05 compared to C1 and conventional gel
Droplet size and Polydispersity index (PDI)
The mean droplet size of the formulations decreased as the concentration of oil decreased the droplet size of the formulations containing different Smix ratio decreased as the surfactant to co-surfactant ratio increased from 1:1 to 1:3. The mean droplet size of C1 formulation with 3.09 % oil having 1:3 Smix was smaller (56.5 ± 4.2 nm) as compared to other formulations (Table 2, Fig 2A1). This might be due to the low oil content in C1 formulation, which leads to the smaller droplet size. The observation was similar to previously reported that the less oil content leads to a decrease in the droplet size25. The PDI of formulation C1 was lower (0.27) as compared to other formulations. The lower values of polydispersity values observed for all the formulations indicated uniformity of droplet size within each formulation.
Zeta Potential
Zeta potential values of all the nanoemulsion formulations are given in Table 2. Zeta potential values of the formulations were found to be in the range - 31.87 to - 7.55 mV. Among the formulations studied C1 showed the highest zeta potential shown in Table 2. Negative values of the zeta potential of the formulations indicated that the formulations were negatively charged and higher value of zeta potential of the C1 formulation signified stability of the system.
Transmission electron microscopy (TEM)
The results of TEM analysis of nanoemulsion C1 correlates with droplet size obtained by PCS and confirmed that droplets of C1 nanoemulsion formulation were spherical in shape and finely distributed with micron size range within 100 nm as shown in Figure 2 B2.
Ex-vivo permeation study
The results of ex-vivo permeation parameters of selected nanoemulsion and control formulation (drug suspension) are illustrated in Table 2. The C1 formulation showed the highest flux (35.67± 3.28 µg/cm2/hr) and significant as compared to other formulation. This might be due to the smaller droplet size which enhanced the permeation of drug through the skin. The results revealed that smaller the droplet size of formulation maximum permeation is observed. Whereas, the A2 formulation had a larger globule size (132.0 ± 2.4nm), showed less flux value (11.05± 3.12 µg/cm2/hr) compared to other formulation. The ex-vivo skin permeation profile of all formulation is shown in Figure 3.
Evaluation of nanogel formulations
Ex-vivo permeation study
The ex-vivo skin permeation results of C1, nanogel, and conventional gel (marketed gel) are shown in Table 2. The nanogel showed the highest flux (50.12 ± 5.12) compared to conventional gel and C1 formulation.
In vivo studies:
Carrageenan induced rat paw edema has been considered as a useful model for studying the anti-inflammatory effect of the drug in rats26. The anti-inflammatory activity outcome of formulations is reported in Table 3, where the value of nanogel indicated a significant difference (P<0.05) as compared to nanoemulsion. The anti-inflammatory activity was found to be 74.0 % after 8 h, which concludes that nanogel performed the greater activity as compared to control group and nanoemulsion. The maximum anti-inflammatory effects of the nanogel formulation could be due to the maximum concentration of ketoprofen available at target side and due to nanosize leads to enhance permeation of drug through the skin.
Stability study
Stability study of the nanogel was carried out for 3 months and no significant change in the values was found in drug content and viscosity as shown in Table 4. This shows that the ketoprofen nanoemulsion nanogel is stable. The stability may be due to the smaller droplet size of nanoemulsion which is less prone to undergo coalescence. The observation is similar to the previously reported27 that smaller droplets are less susceptible to fluctuations which reduced creaming and coalescence, explaining the high stability of nanoemulsions.
CONCLUSION
The study represents the formulation of ketoprofen nanoemulsion loaded gel for transdermal delivery. Based on the globule size (228.8 nm), PDI, zeta potential, flux (10.5±2.6 µg/cm2/hr) formulation C1 was selected as an optimized formulation and converted into gel formulation which gives desirable ex-vivo release profile. The in-vivo study revealed a significant increase in percent inhibition (74.0 % for 8 h) of nanogel formulation as compared (P<0.05) to conventional gel. Overall, the results indicate that developed nanogel formulation can be successfully used for transdermal delivery of ketoprofen in pain management.