INTRODUCTION
Tablets are prepared by compressing unit volumes of granules or a powder blend of the active drug and excipients. Excipients are added in optimal quantities to a formulation to facilitate the tableting process and ensure that the desired quality of the tablet is achieved. For this reason, excipients have been regarded as functional components of a formulation1 because they contribute significantly to the processing, stability, safety, bioavailability and performance of the dosage form containing the drug. Among the many examples available to the pharmaceutical industry, starches constitute a major source of excipients in tablet formulation. They have been drawn from a wide variety of sources including cereal and tuber crops and have been used extensively as diluents, disintegrants, binders and glidants in tablet formulation2,3. Due to their versatility and robust nature, starches can perform several functions in a formulation depending on its mode of incorporation. As a powder, starches can be incorporated as a bulking agent4, intra- or extragranular disintegrant5,6 and glidant4 while as a paste, starches have been utilized as binders7 8-9 in tablet formulation with excellent results. Starches, therefore, have the potential for multifunctional application in tablet formulation. In the present study, maize starch was evaluated as a multifunctional excipient in the formulation of metronidazole tablets by wet granulation. Maize starch was incorporated concurrently as a diluent, disintegrant and binder in metronidazole tablet formulation and compared with a reference formulation containing lactose as diluent, maize starch as disintegrant and acacia as binder using the same concentrations.
MATERIALS AND METHODS
Materials
Metronidazole (Central Drug House (P) Ltd. New Delhi), Maize Starch (Burgoyne Burbidge & Co. (India), Mumbai), Lactose (DFE Pharma, Germany), Acacia (Kerry Ingredients and flavours Ltd, Ireland), Colloidal silicon dioxide (Evonik Industries, Germany), Sodium Stearyl Fumarate (JRS Pharma, Germany). All other materials used were of pharmaceutical grade.
Methods
Preparation of metronidazole granules and tablets
Metronidazole granules were prepared by mixing metronidazole powder (30 g), lactose (filler, 30 g) and maize starch (disintegrant, 7.5 g) together in a mortar and pestle for 5 mins. A binder solution containing acacia (3.75 g) was prepared and incorporated to mass the powder mix. The massed powder was screened by passing through a sieve (1.6 mm). The granules formed were dried in the oven at 40 °C for 10 mins. Subsequently, the granules were passed through sieve (0.8 mm) and dried completely in the oven at 40 °C for 2 h. The entire process of granulation was repeated using maize starch as filler, binder and disintegrant in the same proportions as above. The granules were characterized for particle size, angle of repose, bulk density, tapped density, Carr’s index, Hausner’s ratio and moisture content according to reference methods. The granules were then lubricated by mixing with colloidal silicon dioxide and sodium stearyl fumarate for 5 mins. The lubricated granules were fed automatically into die cavity measuring 12 mm from the hopper and compressed into tablets weighing 500 mg at a compression pressure of 57.5 MPa on a Single Punch Tablet Press (Type EKO, Erweka, Apparatebau, Germany). Tablets were ejected from the die and kept for 24 h to allow for elastic recovery prior to evaluation of tablet properties. The formula for preparing granules and tablet is given in Table 1 below.
Table 1: Formula for preparing metronidazole granules and tablets for batches I & II formulations (batch size=150 tablets)
| Ingredients | I | II |
|---|---|---|
| Metronidazole (40 %) | 30 | 30 |
| Lactose (40 %) | - | 30 |
| Maize starch (40 %) | 30 | - |
| Maize starch (10 %) | 7.5 | 7.5 |
| Maize starch (5 %) | 3.75 | - |
| Acacia (5 %) | - | 3.75 |
| Colloidal silicon dioxide (4 %) | 3 | 3 |
| Sodium stearyl fumarate (1 %) | 0.75 | 0.75 |
| Total (g) | 75 | 75 |
Particle size analysis
The mean granule size (MGS) for each batch of granules was determined using the sieving method10. Test sieves ranging from 1000 µm to < 75 µm (Pan) were arranged in descending order. Approximately 20 g sample of the granules was placed in the top most sieve and allowed to vibrate for 10 mins in the Endecott test sieve shaker to allow for separation of granules based on their different sizes. The quantity of granules retained on each sieve was weighed and the mean granule size was calculated using the formula given below.
Optical microscopy
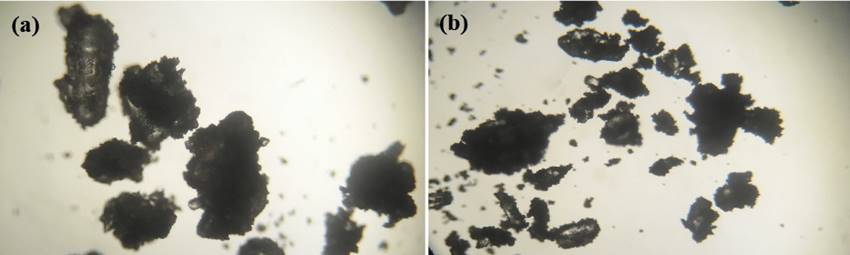
Optical images of the granules examined at 40 x magnification were generated using a camera mounted on the microscope. The photomicrographs for all the batches of granules were taken and recorded.
Angle of repose
The angle of repose for each batch of granules was measured using the Pilpel method11. About 20 g of granules was poured through a funnel suspended at a height of 8 cm onto a flat surface forming a conical heap of powder. The dimensions of height and radius were measured and computed using the equation given below. A mean of three replicates was reported with the standard deviation.
Where h and r are height and radius respectively
Bulk and tapped densities
Bulk (BD) and tapped densities were measured in triplicate for each batch of granules. The volume occupied by 20 g of granules poured into a 50 mL measuring cylinder was recorded as the bulk volume (VB) and the volume obtained after tapping to constant volume was recorded as the tapped volume (VT). Bulk and tapped densities were calculated using the formula given below.
The parameters of Carr’s Index (CI) and Hausner’s ratio (HR) were equally calculated using the bulk and tapped densities as shown in Equations5 & 6 respectively.
Moisture content
The moisture content was obtained by drying to constant weight 1 g sample of granules in the oven at 105 . Moisture content expressed in percentage was calculated using the equation given below,
Uniformity in weight
Twenty (20) tablets from each batch were randomly sampled and their weights determined using an electronic balance. The mean and standard deviation were computed and recorded.
Content uniformity test
The average weight of five tablets powdered was weighed out and dissolved in 100 mL of 0.1 N HCl. The mixture was filtered and sufficiently diluted with 0.1 N HCl before the absorbance value was read off at 277 nm using the UV Spectrophotometer. This was carried out for both batches. The % drug content was computed using the straight-line equation, y = 0.0395x + 0.1314, derived from the calibration curve of metronidazole where y is the absorbance and x is drug concentration (µg/mL).
Tablet thickness
The thickness of five (5) tablets randomly selected from each batch was measured using a digital vernier calliper. A mean of five determinations was recorded with standard deviation.
Crushing strength (CS) and tensile strength (TS)
The force required to a crush a tablet diametrically was measured using a Monsanto hardness tester. A mean of five replicates was recorded as the crushing strength for each batch of tablet. Tensile strength was calculated using the Fell and Newton13 equation given below:
Where F, d and t are the crushing strength, diameter and thickness respectively.
Friability test (FR)
Tablet friability was determined for each batch of tablets using an Erweka Friabilator (TA 20, GmbH, Heusenstamm, Germany). The initial weight (Wi) of ten (10) tablets was taken, placed in the friabilator and allowed to rotate at 25 rpm for 4 min. The final weight (Wf) of tablets was taken after removal of dust and friability calculated as follows:
Disintegration time (DT)
The time taken for six (6) tablets to disintegrate from each batch was assessed using the Disintegration tester (ZT-4, Erweka, Heusenstamm, Germany) as described in the British Pharmacopoeia12. A mean of six determinations was recorded for each batch of tablets.
In vitro drug release studies
Tablet dissolution studies was conducted on both batches using the Erweka dissolution apparatus (Type DT6, GmbH, Heusenstamm, Germany) under sink conditions. Drug release was determined in 900 mL 0.1 N HCl at a basket speed of 50 rpm and temperature of 37 ± 0.5°C. A tablet was placed carefully in the vessel and at the following intervals of 5, 10, 20, 30, 45 and 60 mins respectively, 5 mL samples were withdrawn and replaced with equal volume of 0.1 N HCl after each withdrawal. The samples collected were filtered and sufficiently diluted with 0.1 N HCl before taking the absorbance readings at 277 nm using the UV spectrophotometer (UV - 1800 Spectrophotometer, Shimadzu Corporation, USA). The amount of metronidazole released (%) was calculated based on the regression equation, y = 0.0395x + 0.1314, derived from the calibration curve of metronidazole. A mean of three determinations was obtained and a graph of amount of drug released (%) against time was plotted to establish the dissolution profile of both batches of tablets.
RESULTS
Granule Properties
The granule properties of batches I & II formulations are presented in Table 2. The mean granule size (MGS) for batch I formulation was greater than that of batch II. Evaluation of flow parameters revealed that the angle of repose for both batches ranged between 32 - 36 º. A higher flow rate was obtained for batch I formulation relative to batch II formulation.
Table 2: Granule properties of Batches I & II formulations
| Parameters | Batch I | Batch II |
|---|---|---|
| Mean granule size (µm) | 330.46 | 288.35 |
| Angle of repose(ᶱ) | 36.04 ± 1.22 | 32.17 ± 2.62 |
| Bulk density (g/mL) | 0.41 ± 0.002 | 0.45 ± 0.00 |
| Tapped density (g/mL) | 0.49 ± 0.01 | 0.56 ± 0.00 |
| Flow rate (g/sec) | 4.26 ± 0.17 | 3.71 ± 0.0 |
| Carr’s index (%) | 17.47 ± 1.27 | 19.1 ± 0.0 |
| Hausner’s ratio | 1.21± 0.02 | 1.24 ± 0.0 |
| Moisture content (%) | 5 | 7 |
Bulk and tapped densities of batch I formulation lower in comparison to batch II formulation. The values of CI and HR computed for batch I formulation were slightly lower than those of batch II formulation. Moisture content for both formulations did not exceed 7 %. Photomicrographs of batches I & II formulations displayed as Figure 1 shows that the granules of batch I formulation are bigger in size compared to granules of batch II formulation. The granules for both formulations appear irregular and spongy-like in shape demonstrating a high degree of agglomeration of primary powder particles. Overall, granule properties for both formulations appear to differ considerably possibly due to the composition of the formulation.
Tablet Properties
Tablet properties of both formulations are summarized in Table 3. The mean tablet weight of batches I & II formulations was 498 and 494 mg respectively. Content uniformity of batch I tablets was above the limit set for acceptable tablets compared to batch II tablets. Thickness of the tablets varied with the batches as batch I tablets recorded a lower mean thickness compared to that of batch II. Tablet density was slightly higher for batch I tablets compared to batch II tablets.
Table 3: Tablet properties of batches I & II formulations
| Parameters | Batch I | Batch II |
|---|---|---|
| Mean weight (mg) | 498 ± 3.40 | 494 ± 5.03 |
| Content uniformity (%) | 113.35 | 103.87 |
| Thickness (mm) | 4.46 ± 0.01 | 4.84 ± 0.1 |
| Tablet density (g/mL) | 0.99 ± 0.01 | 0.87 ± 0.01 |
| Crushing strength (N) | 42.6 ± 2.1 | 20 ± 1.7 |
| Tensile strength (MPa) | 0.5 ± 0.02 | 0.22 ± 0.02 |
| Friability (%) | 1.41 | 3.63 |
| Disintegration time (min) | 3.75 ± 0.51 | 0.22 ± 0.0 |
The crushing strength of batch I tablets was found to be higher compared to that of batch II. This correlated well with the friability and disintegration times of tablets as the batch of tablets with higher crushing strength produced tablets that were less friable and took a longer time to disintegrate into granules and primary particles. The drug-release profile of both formulations illustrated in Figure 2 shows that more than 80 % of the drug was released in less than 5 mins for both formulations.
DISCUSSION
The aim of this study was to evaluate the performance of maize starch as a multifunctional excipient in the formulation of metronidazole tablets by wet granulation. A multifunctional excipient is that excipient that can serve two or more roles in a formulation14,15 i.e. diluent, disintegrant and binder. Maize starch was utilized as a diluent, disintegrant and binder in batch I formulation in comparison to its performance as a disintegrant only in batch II formulation. Significant differences were observed in the granule properties of both formulations suggesting that the composition of the granules exerted a dominant effect on its properties. Mean granule size (MGS) of batch I formulation was greater than that of batch II formulation owing to the pasting and binding properties of maize starch. In addition, maize starch present in large proportions as a multifunctional excipient in batch I formulation may have contributed to the wettability of the powder blend of ingredients leading to a greater degree of agglomeration of primary powder particles during granulation. The swelling property of starch attributed to its hydrophilicity is likely to have facilitated particle adhesion during granulation leading to granules larger in size. The extent of wettability of acacia as a binder in batch II formulation may have influenced its capacity to agglomerate particles during granulation giving rise to a MGS relatively lower than that of batch I formulation. Assessing the flow properties of the granules showed that the flow rate of granules was consistent with the granule size as larger particles generally flow faster. Hence batch I formulation had a better flow rate compared to batch II formulation. Similarly, bulk and tapped densities of batch I granules were relatively lower compared to that of batch II granules because of lower degree of packing associated with larger particle size and this translated to better flow rate due to a greater degree of porosity. The granule properties obtained for batch I formulation translated into better tableting properties for the formulation in terms of crushing strength, tensile strength, tablet density and friability. The differences observed in the tableting parameters of crushing strength and disintegration time of both formulations was statistically significant at p < 0.05. The higher crushing and tensile strength values obtained for batch I formulation can be attributed to a greater degree of bonding area and bonding strength per unit area occurring during compression. It implies therefore that the formulation containing maize starch as a multifunctional excipient was more compressible and compactable leading to a better tabletability of the formulation. This is consistent with the findings of Nasipuri16 and Deshpande and Panya17 who recorded better performances in tableting properties when starches from various sources were used as binders in comparison to acacia. The poor friability outcome of both formulations can be attributed to a higher concentration of disintegrant (10 %) used in the formulation compared to a binder concentration of 5 %. The relatively longer disintegration time of batch I formulation can be linked to the hardness of the tablets. Harder tablets take a longer time to disintegrate as it will require more time for the disintegrating medium to overcome the strength of the bonds formed in the tablet. Nevertheless, both formulations passed the disintegration test as the disintegration time did not exceed 15 mins as recommended for immediate release tablets12. The drug-release profile of both formulations did not differ significantly even though a significant difference (p < 0.05) was observed in their disintegration times. Maximum drug release of 100 % was attained at about the same rate and time for both formulations. This agrees with the findings of Odeku and Akinwande6assessed using the disintegration and dissolution (t 50 and t 80 - time required for 50% and 80% of paracetamol to be released that the dissolution rate does not necessarily depend on the disintegration time.
CONCLUSION
The application of maize starch as a multifunctional excipient has been investigated. Better tableting properties were obtained with formulations containing maize starch as multifunctional excipient compared to when it was used only as a disintegrant in batch II formulation. This confirms the suitability of maize starch as a multifunctional excipient in tablet formulation.