Introduction
Avocado oil (Persea americana) consists mainly of fatty acids1 where oleic acid its major component1,2. Carotenoids, tocopherols, sterols and chlorophylls are also found in is its composition1. Regarding to skin, avocado oil has properties such as antioxidant, anti-aging, anti-inflammatory, and healing3,4. Avocado crude oil is more advantageous than refined crude oil in relation to its skin benefits. Nevertheless, refined avocado oil is the one most used in skin formulations5. Under exposure to daylight and thermal exposure, crude avocado oil components suffer degradation, which compromises its skin properties6. Also, in the refining process, chlorophylls are removed allowing the obtaining of an refined avocado oil which is less susceptible to oxidation by light1.
Drug delivery systems have been widely described as cosmetics and pharmaceutical carriers7-9. Nanocapsules are vesicular systems containing a polymeric membrane and an oily core10. Since these carriers have shown instability during storage, it is primordial to assess its stability. Moreover, there is a trend to the use of vegetable oils as ingredients of nanocapsules oily core as an alternative to synthetic oils11-15. As there is a great worldwide biodiversity, the use of vegetable ingredients should be encouraged in skincare products.
This study aimed to develop crude avocado oil-loaded nanocapsules to obtain an aqueous dispersion with suitable stability. To the best of our knowledge, there are no reports describing crude avocado oil as an oily-core component of nanocapsules. Most of the reports regarding avocado oil are intended for food applications for the nutritional supply of vitamins and fatty acids that are beneficial to health. The consumption of this food is associated with the reduction of the inflammatory process, improvement of the cardiovascular condition and reduction of oxidative stress induced by diabetes2.
In this work, three distinct nanocapsules formulations with variations in its ingredients content were evaluated after prepare and throughout storage. Avocado oil has a dual purpose in those nanoformulations: as a component of nanocapsules oily core and as an active ingredient in cosmetic or dermatological therapy.
Methods
Avocado oil-loaded nanocapsules (NCAO) preparation
Crude avocado oil-loaded nanocapsules dispersions were prepared by interfacial deposition of the pre-formed polymer method16. Three aqueous dispersions were prepared and named as NCAO-1, NCAO-2 and, NCAO-3. Variations in polymer and oil amounts were accomplished in each of these aqueous formulations. The other ingredients were kept constant in all formulations (Table 1). The organic phase was composed of poli-ε-caprolactone molecular weight 65,000 (Sigma-Aldrich, USA), crude avocado oil (donation from Avocado Br, Brazil), sorbitan monostearate (Sigma-Aldrich, USA) and acetone (Vetec, Brazil). The aqueous phase was composed of polysorbate 80 (ALZ, Brazil) and 270 mL of water. A final volume of 50 mL was achieved after the removal of acetone and part of water by evaporation under pressure. All aqueous dispersions were prepared in triplicate.
Table 1. Amount of ingredients in avocado oil-loaded nanocapsules dispersions.
| Dispersion | Poly-ε-caprolactone (mg/mL) | Avocado oil (% w/v) | Sorbitan monostearate (mg/mL) | Polysorbate 80 (mg/mL) |
|---|---|---|---|---|
| NCAO-1 | 10 | 3.5 | 7.66 | 7.66 |
| NCAO-2 | 20 | 5.0 | 7.66 | 7.66 |
| NCAO-3 | 20 | 7.0 | 7.66 | 7.66 |
Avocado oil-loaded nanocapsules characterization and stability.
Nanocapsules were analysed by pH, particle size, and polydispersity index [PDI] and zeta potential measurements. pH from dispersions was determined in a calibrated potentiometer (Metler Todelo, Brazil)15-17. Particle size and PDI were accomplished by photon correlation spectroscopy (Zetasizer NanoSeries, UK)8. Zeta potential analysis performed by electrophoretic mobility (Zetasizer NanoSeries, UK) 12,17)). Stability studies were performed after one and two months of aqueous dispersions storage by measurements of pH, particle size, zeta potential, and PDI. The most stable formulation was analysed after six months of storage. All formulations employed in stability studies remained in ambient tempera ture. Analyses were performed in triplicate.
Assessment of avocado oil oxidative stability
Avocado oil sample named as standard avocado oil was protected from daylight and heat. This sample was evaluated regarding the acid index and peroxide index to ensure its quality18. The average acid index and average peroxide index were 1.55 mg KOH g-1 and 2.9 mEq O2 /Kg, respectively. As these values are following a report of Jorge et al., 201519, avocado oil quality was ensured.
Furthermore, avocado-oil loaded nanocapsules as well as standard avocado oil were evaluated by gas chromatography (GC). 5 mL of nanocapsules dispersions were treated with 250 mL of acetonitrile (Vetec, Brazil) causing polymeric membrane dissolution and the release of avocado oil from nanoparticles. The obtained solution was submitted to liquid-liquid extraction with 50 mL of petroleum ether (Vetec®, Brazil). The resulting organic phase was subjected to petroleum-ether extraction. This extraction was repeated three more times. Ultimately, ether fractions were pooled and concentrated under reduced pressure at 40 ° C to allow petroleum ether evaporation. The remaining vegetable oil was submitted to transesterification using boron trifluoride in methanol (BF3) (Merck, Germany)18. After transesterification, 0.2 uL of samples from standard avocado oil, NCAO-1, NCAO-2, NCAO-3 were injected in a gas chromatography (Varian 3300), using a DB-5 column, 30m long x 0.53mm diameter (Agilent, USA) and nitrogen as mobile phase. Flame ionization detector was composed of Hydrogen gas and synthetic air. Detector and injector temperatures were set in 210ºC and 270ºC, respectively. Oven temperatures ranged from 180ºC to 250ºC with a rate of 5º C per minute. Inlet temperature was sustained for 0.1 minutes.
Results
All formulations were homogeneous with a milky white appearance. Results of particle size, PDI, zeta potential, and pH of nanocapsules formulations after preparation are shown in Table 2. Nanocapsules had particle size ranging from 258 to 447 nm on average. Aqueous dispersions had differences (p< 0.05) concerning particle size. NCAO-1 had the smallest particle size while NCAO-3 had the largest particle size. Aqueous dispersion NCAO-2, on the other hand, had a particle size larger than NCAO-1 and smaller than NCAO-3.
Regarding PDI values, NCAO-1 presented the lowest value close to 0.2. As for NCAO-2 and NCAO-3, values were approximately 0.42 and 0.56, respectively. PDI values followed the same behaviour as that observed for particle size. Aqueous dispersion NCAO-1 had the lowest values. Diversely, aqueous dispersion NCAO-2 had higher size values than NCAO-1, but lower PDI than NCAO-3 (p <0.05). As for pH values, aqueous dispersions NCAO-1 and NCAO-2 had a similar value close to 5.8 (p>0.05). However, aqueous dispersion NCAO-3 presented slightly more acid values (p <0.05). In respect to zeta potential, all dispersions had negative zeta potential, around -13 mV on average, and were not statistically different (p> 0.05).
Table 2. Particle size, PDI and zeta potential of avocado oil-loaded nanocapsules after preparation (mean±sd).
| Dispersion | Particle size(nm) | PDI | Zeta potential (mV) | pH |
|---|---|---|---|---|
| NCAO-1 | 258.65±8.08 C a | 0.22±0.02C a | -13.82±1.78 | 5.81 ±0.23A a |
| NCAO-2 | 359.62±18.31B a | 0.42±0.05B a | -13.55±1.32 | 5.76±0.07A a |
| NCAO-3 | 447.93±20.77 A a | 0.56±0.09A a | -13.34±1.17 | 5.59±0.10C a |
| p-value | <0.0001 | <0.0001 | 0.9222 | 0.0010 |
a. Means followed by same letters in columns are not considered different in Tukey's test.
The Formulations mantained the white milky colour during 2 months of storage, without precipitation or sedimentation. Table 3 below displays results of size, PDI, and zeta potential of nanocapsules dispersions after 1 month of storage. Concerning particle size, dispersions with the highest amount of polymer and oil (NCAO-2 and NCAO-3) presented the largest particle size (p> 0.05). As for PDI, the values increased in the following order: NCAO-3> NCAO-2> NCAO-1. With reference to zeta potential, more negative values were obtained for NCAO-2 (p <0.05) while the less negative values were obtained for NCAO-3. The dispersion with less oil and polymer content had intermediate zeta potential values.
Table 3. Particle size, PDI and zeta potential of avocado oil-loaded nanocapsules dispersions after 1 month of storage (mean±sd).
| Dispersion | Particle size(nm) | PDI | Zeta potential (mV) | pH |
|---|---|---|---|---|
| NCAO-1 | 317.83±47.49B a | 0.35±0.12C a | -13.43±1.49B a | 4.96±0.28 |
| NCAO-2 | 426.97±35.96A a | 0.47±0.08B a | -16.91±2.12A a | 5.10±0.22 |
| NCAO-3 | 412.07±42.94A a | 0.53±0.10A a | -11.76±1.08C a | 5.05±0.21 |
| p-value | <0.0001 | 0.0024 | <0.0001 | 0.4559 |
a. Means followed by same letters in columns are not considered different in Tukey's test.
Table 4 shows the average results from aqueous dispersions after two months of storage. The smallest particle size was obtained for NCAO-1 while or, the dispersions NCAO-2 and NCAO-3 exhibited the largest particle size. However, NCAO-2 and NCAO-3 were not different from each other. For PDI values, similar mean values of was 0.45 obtained for the dispersions NCAO-2 and NCAO-3, and NCAO-1 had the lowest PDI values. Regarding zeta potential, NCAO-1 value had the most negative values and formulations NCAO-2 and NCAO-3 were not different. There were no differences in pH (p <0.05).
Table 4. Particle size, PDI and zeta potential of avocado oil-loaded nanocapsules dispersions after 2 months of storage (mean±sd).
| Dispersion | Particle size(nm) | PDI | Zeta potential (mV) | pH |
|---|---|---|---|---|
| NCAO-1 | 311.49 ±49.94B a | 0.32±0.09B a | -13.74 ± 1.97A a | 4.61±0.22 |
| NCAO-2 | 458.22±102.63 A a | 0.45±0.05A a | -11.12 ± 0.96B a | 4.58±0.18 |
| NCAO-3 | 426.94 ± 87.39A a | 0.45±0.09A a | -11.03 ± 0.82B a | 4.76±0.32 |
| p-value | 0.0032 | 0.0072 | 0.0003 | 0.2746 |
a. Means followed by same letters are not considered different in Tukey's test.
Since NCAO-1 had the best stability, this formulation was prepared again and analysed after six months of storage (Figure 1). An increase in particle size (from 248 nm to 296 nm) and in PDI values (from 0.22 to 0.29) was observed. Regarding pH, there was acidification of the dispersion. For zeta potential, the values ranged from -10 mV to -13 mV, indicating that zeta potential became even more negative after six months of storage. For all parameters analysed, statistical differences were observed (p <0.05).

Figure 1. Dispersion NCAO-1 evaluation after preparation and after six months (mean±sd). Means followed by same letters were considered as not significant different in the Tukey's test.
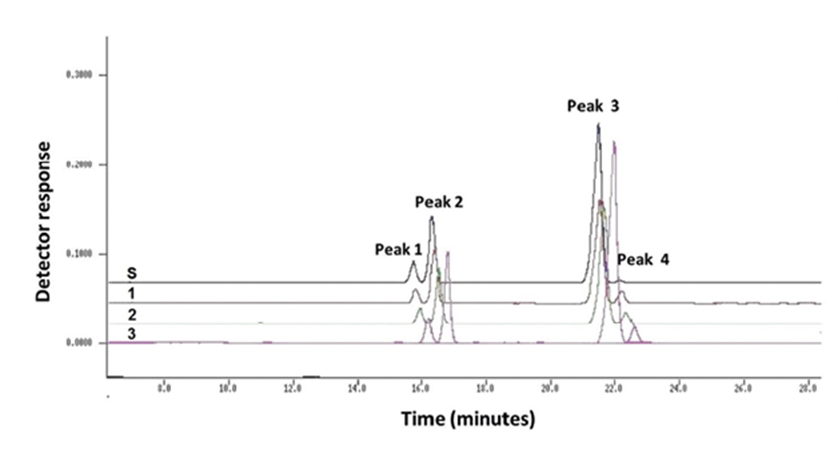
The chromatographic profile of avocado oil from nanocapsules and standard avocado oil is in Figure 2. Avocado oil from nanocapsules and standard showed a similar chromatographic profile. Each chromatogram had 4 retention peaks in 16, 17, 22 and 22.5 minutes, respectively.
Discussion
Aqueous dispersions presented particle size ranging from 250 nm to 447 nm according to nanocapsules prepared by interfacial deposition10. Due to the high polymer and oil amount20, NCAO-2 showed a slight precipitate during its preparation. Furthermore, the highest particle size obtained for aqueous dispersions NCAO-2 and NCAO-3 is explained by their highest polymer and oil amounts13. Moreover, the higher the amount of polymer and avocado oil the larger particle size obtained. Accordingly, NCAO-3 had the highest particle size. Aqueous dispersion with the lowest content of oil/polymer (NCAO-1) presented the lowest particle size and lowest PDI, indicating a narrow particle size distribution21. As for NCAO-2 and NCAO-3 PDI, values indicate a broad particle distribution22.
Zeta potential predicts formulation stability and is influenced by polymer, surfactants nature, pH of the medium10. All formulations had negative potential zeta according to previous reports15,20. Negative values are explained by the presence of polymer Poly-ε-caprolactone and the presence of surfactant polysorbate 8020. Although the formulations had a difference in its quantitative composition, it did not affect the zeta potential since there no statistical differences between them.Though dispersion NCAO-3 had a slightly more acid pH, this factor also did not affect its zeta potential.
Analyses of pH are important to check the compatibility of the formulation developed with the skin. All nanocapsules prepared had appropriate pH values for skin applications17and according to nanocapsules prepared by interfacial deposition of pre-formed polymer17,21. Besides, PCL is susceptible to hydrolysis, which causes a reduction in the pH of nanocapsules dispersions 15,23 . This lower pH, not physiological, can cause skin irritations or injuries. Therefore, the formulation with the lowest ingredients (NCAO-1) shows the most physicochemical stability.
Colloidal system stability assessment is an essential phase of new product development since significant changes in stability can affect formulation efficacy and safety24. In this context, analyses performed during storage showed that both NCAO-2 and NCAO-3 presented the highest particle sizes. This result is in agreement with the initial characterization where these dispersions also presented the largest particle. The largest particle indicates aggregation and consequently low stability. Besides, the high standard deviation observed for NCAO-2 and NCAO-3 dispersions particle size may be due to their dynamic behaviour20.
Concerning PDI, the highest PDI values were expected to dispersions NCAO-2 and NCAO-3. An excess of oil and polymer could also be present in these nanocapsules formulations which could induce the formation of other nanostructures25 which affects particle size homogeneity20,25 and consequently PDI values. Furthermore, the oil in excess may not be fully encapsulated and may easily be oxidized. As for pH, no significant changes (p> 0.05) were observed either after one month or after two months of storage. Changes in pH during storage are due to polymer hydrolysis16. Then, in our work, the polymeric hydrolysis could be at least partially reduced or avoided. When polymer hydrolisis occurs, the polymeric shell strcucture is compromised whic affects affects physicochemical stability.Small variations in zeta potential (p <0.05) were observed after one and two months of storage for dispersions NCAO-2 and NCAO-3. These variations may be due to dynamic behavior which negatively impacts stability of these formulations.Under the tested conditions, nanocapsules dispersion NCAO-1 demonstrated the best stability and therefore is suitable for further studies. Although some variations were observed after six months of storage this instability is not a limiting factor. As this dispersion is intended for cutaneous application, its addition in semisolid vehicles is required. An increase in the viscosity of final formulation is then achieved which contributes to increase the stability of final formulation. Furthermore, this aqueous dispersion has showed adequate stability after its preparation. Therefore, the dispersion must be added in a semisolid vehicle shortly after its preparation.
Regarding chromatographic profile, since there is a greater amount of oil in NCAO-2 and NCAO-3 we expected significant changes in GC due to oil oxidation. Nevertheless, no sign of changes in the GC profile was observed for any of the nanocapsules dispersions. Furthermore, avocado oil is photolabile, unless protected from light and heat26. Avocado oil loaded in nanocapsules had a similar chromatographic profile to standard avocado oil, no thermal oxidation or photo-oxidation was detected.
In the development of new drug delivery systems, characterization and stability tests are essential to understand their behaviour. Further studies should also be employed to assure a more complete characterization and a proper stability of nanoparticles. After a global analysis, the delivery system is then suitable to be added into a semi-solid form. Despite their preliminary assessment, avocado crude oil-loaded nanocapsules represent a complementary treatment of atopic dermatitis, psoriasis and for increasing skin healing in diabetic patients.
Conclusion
Nanocapsules aqueous dispersions with the lowest oil and polymer amount showed the best physicochemical stability after two months of storage. Avocado oil from aqueous nanocapsules did not show evidence of oxidation. This work showed preliminarily the stability of crude avocado oil-loaded nanocapsules dispersions. Further studies of characterization and stability of nanocapsules dispersions are required.Then, skin formulation containing avocado oil nanoparticles can be developed and evaluated regarding to its efficacy on the skin.