Puntos clave
A pesar de que llevamos casi más de un año de pandemia, continuamos sin tratamientos eficaces para el tratamiento del coronavirus, con la excepción de los corticoides y algunos trabajos recientemente publicados de tocilizumab.
Es por este motivo que parece fundamental la investigación y publicación de los resultados obtenidos con los diferentes tratamientos empleados.
Introducción
SARS-CoV-2 coronavirus infection could be manifested in most patients asymptomatically or with mild symptoms (fever, dry cough, myalgia, headache, diarrhea, anosmia, among others). Other patients develop a severe lower respiratory tract infection, with significant dyspnea requiring oxygen therapy, which can progress into a life-threatening acute respiratory distress syndrome1.
Tocilizumab is a monoclonal antibody that binds to the interleukin 6 (IL6) receptor, and it is used in rheumatoid arthritis2.It has been used off-label, in the absence of specific treatments, in SARS-CoV-2 coronavirus infection, to block the action of IL6, and to decrease the hyperinflammatory response in severe patients.
In this article we present the use of tocilizumab in a Spanish hospital during the first wave of the COVID-19 pandemic.
Métodos
Retrospective cohort study conducted in a 258-bed hospital, 12 of which were Intensive Care Unit (ICU) beds; from February 26 to May 20, 2020.
All SARS-CoV-2 infected subjects diagnosed by a positive RT-PCR test of nasopharyngeal swab or sputum, admitted to the hospital during the study period were included. Those under 18 years of age, pregnant or breastfeeding women were excluded. Data from readmitted patients were also excluded from the analysis. The clinical characteristics of the study sample have been published recently3. Subsequently, we selected tocilizumab-treated subjects with moderately severe infection (PaO2/FiO2 [PAFI] < 300). Patients treated with other anti-inflammatory drugs such as anakinra and/or baricitinib were excluded.
The primary endpoint was mortality rate, while the secondary endpoint was treatment success, considering as the opposite of failure defined as death or ICU admission (PAFI < 200).
Demographic data, comorbidities, clinical symptoms prior to admission, laboratory test and radiological results, as well as treatments during hospital admission were obtained. The patients included in the study gave their consent to receive treatment according to the local protocol, and the study was approved by the corresponding IRB on May 25, 2020. Adverse effects related to tocilizumab were also recorded.
Qualitative variables were described as frequencies and percentages, and analyzed by Chi-square test or Fisher’s exact test as appropriate. Quantitative variables were described as means or medians and interquartile range (IQR); and analyzed parametrically or non-parametrically, according to their normal distribution. The impact measures used were the Odds Ratio (OR) for outcome, death and success, and were obtained adjusted for age, sex and corticosteroid dose by logistic regression. For laboratory parameters before and after drug administration, Student’s t-test for paired samples was used. All tests were considered significant with an alpha of 0.05; and the IBM SPSS statistical package was used (IBM SPSS Statistics for Windows, Version 19.0. Armonk, NY: IBM Corp.).
Resultados
Patients treated with one or more anti-inflammatory drugs (four with baricitinib; 26 with anakinra were 11.4% of the 255 patients infected with SARS-CoV-2 ). Of the remaining 226, 18 subjects received tocilizumab, and 39.4% (n=89) had a PAFI<300. The main characteristics of the sample are described in Table 1.
Table 1. Characteristics of patients with PaO2/FiO2 < 300 at hospital admission.
| N (%) | NT Group (n= 71) | T Group (n= 18) | p |
|---|---|---|---|
| Age in years, median (IQR) | 78,1 (68,1-84,6) | 67,2 (59,0-75,4) | 0,01 |
| Sex Female Male | 32 (45,1) 39 (54,9) | 8 (44,4) 10 (55,6) | 0,96 |
| Hypertension | 45 (63,4) | 8 (44,4) | 0,14 |
| Diabetes | 27 (38,0) | 3 (16,7) | 0,10 |
| Ischemic heart disease | 8 (11,3) | 1 (5,6) | 0,68 |
| Chronic kidney disease | 21 (29,6) | 2 (11,1) | 0,14 |
| Chronic obstructive pulmonary disease | 4 (5,6) | 3 (16,7) | 0,14 |
| Asthma | 5 (7,0) | 3 (16,7) | 0,20 |
| Other chronic pulmonary diseases | 9 (12,7) | 2 (11,1) | 1,00 |
| Heart failure | 6 (8,5) | 1 (5,6) | 1,00 |
| Cirrhosis | 1 (1,4) | 1 (5,6) | 0,37 |
| Cancer | 11 (15,5) | 2 (11,1) | 1,00 |
| Cardiovascular disease | 20 (28,2) | 3 (16,7) | 0,38 |
| Cerebrovascular disease | 6 (8,5) | 0 (0,0) | 0,34 |
| Dyslipidemia | 35 (49,3) | 7 (38,9) | 0,43 |
| Smoker | 8 (11,3) | 1 (5,6) | 0,68 |
| Obesity (BMI≥30 Kg/m2) | 16 (22,5) | 5 (27,8) | 0,64 |
| HIV | 1 (1,4) | 0 (0,0) | 1,00 |
| Inflammatory bowel disease | 0 (0,0) | 0 (0,0) | -- |
| Autoimmune diseases | 4 (5,6) | 2 (11,1) | 0,60 |
| Dementia | 11 (15,5) | 0 (0,0) | 0,11 |
| Pulmonary embolism or deep vein thrombosis | 1 (1,4) | 0 (0,0) | 1,00 |
| Rheumatoid arthritis | 4 (5,6) | 1 (5,6) | 1,00 |
| Treatment | |||
| Anticoagulant | 15 (21,1) | 0 (0,0) | 0,04 |
| NSAIDs | 4 (5,6) | 1 (5,6) | 1,00 |
| ACE inhibitors or ARBs | 34 (47,9) | 6 (33,3) | 0,27 |
| Symptoms | |||
| Fever | 53 (74,6) | 16 (88,9) | 0,20 |
| Dyspnea | 35 (49,3) | 10 (55,6) | 0,64 |
| Dry Cough | 36 (50,7) | 15 (83,3) | 0,02 |
| Expectoration | 9 (12,7) | 5 (27,8) | 0,12 |
| Sore throat | 2 (2,8) | 0(0,0) | 1,00 |
| Myalgia | 10 (14,1) | 4 (22,2) | 0,47 |
| Headache | 5 (7,0) | 2 (11,1) | 0,63 |
| Dizziness | 2 (2,8) | 2 (11,1) | 0,18 |
| Diarrhea | 14 (19,7) | 5 (27,8) | 0,46 |
| General malaise | 37 (52,1) | 8 (44,4) | 0,56 |
| Anosmia | 2 (2,8) | 0 (0,0) | 1,00 |
| Ageusia | 2 (2,8) | 0 (0,0) | 1,00 |
| Chest pain | 2 (2,8) | 1 (5,6) | 0,50 |
| Days with symptoms, median (IQR) | 7,0 (3,0-8,0) | 5,5 (2,5-7,0) | 0,37 |
IQR: interquartile range. T group: tocilizumab-treated group. NT group: group not treated with tocilizumab.
No differences were found in the radiological pattern between the tocilizumab-treated group (T group) versus the (NT group) (p=0.82).
The T group presented fewer days with symptoms (5 days vs 7 days) and received a mean dose of 666.7 mg (SD=72.31). The T group had also a significantly lower (p=0.01) minimum PAFI during hospitalization, with a median of 147.5 (95%CI: 116.7 to 194.0) versus 255.6 (95%CI: 320.7 to 452.4) in the NT group. The T group received on average 90.4 (SD=20.8) mg of methylprednisolone and the NT group 58.9 (SD= 9.48) mg on average. The median duration of corticosteroid therapy was 7 days (95%CI: 3 to 11 days) and 3 days (95%CI: 3 to 6 days) for T and NT group, respectively.
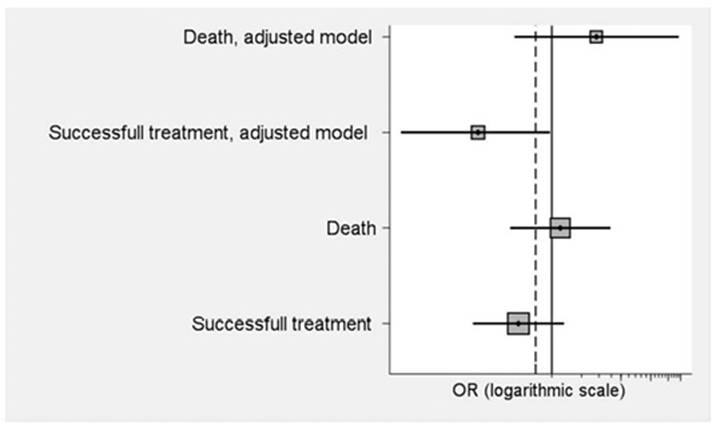
In the T group died 5 patients (27.8%), and 17 (23.9%) in the NT group without significant differences between the two groups (p=0.74). The OR was 1.22 (95%CI: 0.38 to 3.92). No differences were also observed (p=0.15) in terms of treatment success (n=10; 55.6%) or not success (n=52; 73.2%) in the T group, with an OR=0.46 (95%CI: 0.16 to 1.33). Figure 1 shows the crude ORs, and adjusted ORS for age, sex and total corticosteroid dose of the final results. After adjustment, the OR of successes obtained becomes significant (p=0.05), being 0.18 (95%CI: 0.03 to 0.96), while it is still not significant in mortality.
Table 2 shows the laboratory results before and after tocilizumab administration, significantly affecting C-reactive protein values (p=0.04). In T group the most frequent adverse effects were moderate neutropenia (500-1000 neutrophils/ µL) in 3 patients (16.6%), and increased transaminases (3 times the upper normal limit) in 4 patients (22.2%).
Table 2. Evolution of inflammatory analytical parameters before and after tocilizumab administration.
| Laboratory test, median (IQR) | Tocilizumab (N=18) | p | |
|---|---|---|---|
| Before | After | ||
| Leukocytes, absolute count x 109/L | 6,3 (3,9-7,9) | 7,7 (3,7-11,9) | 0,30 |
| Lymphocytes, count x /L | 0,6 (0,5-1,2) | 1,2 (0,7-1,4) | 0,15 |
| C-reactive protein, mg/dL | 11,30 (5,29-18,03) | 1,74 (0,26-7,51) | 0,04 |
| Lactate dehydrogenase, U/L | 369,0 (258,0-382,5) | 461,0 (222,0-531,5) | 0,47 |
| D-dimer mg/mL | 883,0 (780,5-2147,5) | 1246,5 (534,0-2931,0) | 0,31 |
| Ferritin, ng/mL | 963,8 (741,5-2001,4) | 1265,3 (859,0-2198,4) | 0,13 |
| Procalcitonin ng/mL | 0,140 (0,110-0,199) | 0,121 (0,049-0,242) | 0,35 |
| Aspartate aminotransferase (AAT), U/L | 63,1 (44,6-109,2) | 63,1 (44,6-70,3) | 0,37 |
| Alanine aminotransferase (ALT), U/L | 29,5 (16,4-37,9) | 38,2 (21,3-71,2) | 0,17 |
| Troponin T-hs, pg/mL | 11,98 (8,87-18,01) | 9,73 (8,50-21,87) | 0,32 |
| PaO2/FiO2 | 306,7 (240,7-337,5) | 356,5 (156,8-445,2) | 0,78 |
Discusión
In our cohort of patients with moderately severe SARS-CoV-2 infection, tocilizumab administration did not produce improvement in neither mortality nor in health care success. Perhaps, it could improve mortality as showed in Figure 1, but due to the small cohort size this is inconclusive.
These results are in concordance with other studies where tocilizumab showed no significant improvement in mortality rates4,5. However, other results suggested that tocilizumab could enhance survival6-8),
These differences could be due to the studies design or the moment in the pandemic in which they were conducted. In a recent published meta-analysis, the authors found significant differences according to the study design9
Our low sample size is due to drug supply problems, and to the restrictions imposed by the Ministry of Health10. During the first COVID-19 pandemic wave, tocilizumab treatments were administered to the most critical patients, and to those who were not responding to corticosteroids and high oxygen flow treatment.
Subjects in the T group were significantly younger than those in the NT group, showing a possible selection bias. A non-significant improvement in respiratory status with higher PAFI (see Table 2) after tocilizumab administration, such as in previous studies, was observed4
Tocilizumab produced improvement in the hyperinflammatory state, reducing C-reactive protein by 84.6% after administration, a similar result to that obtained by Albertini et al11. No differences were found in D-dimer and ferritin as observed by other authors.8,12,13
Tocilizumab was well-tolerated, and, from a safety point of view, the adverse effects observed (neutropenia and hepatotoxicity) were transient. Similar results were obtained by Duarte et al7 in a cohort of elderly patients in our country.
Dexamethasone has been shown to be lifesaving treatment for moderate COVID-19 infections14. The results of the RECOVERY study12 have recently been published showing a benefit in mortality after adding tocilizumab to corticosteroids, improving the absolute risk difference by 4%. Its marginal benefit or whether it is due exclusively to the corticosteroid is not entirely clear. In our study, we considered it a confounding factor, and therefore, we performed an adjusted analysis, as well as by sex and age, without significant results.
This work presents the limitations of retrospective observational studies (selection bias and confounding factors). Consequently, our results should be taken with caution, and should be confirmed in randomized clinical trials.
In conclusion, although we did not detect significant differences in survival, the use of tocilizumab in moderately severe hospitalized COVID-19 infected patients could reduce or alleviate the hyperinflammatory state, preventing disease progression and admission to the ICU.





![Dolor lumbar persistente tras la administración de [131I]Iodo-6-β-iodometil-19-norcolesterol: a propósito de un caso](/img/es/next.gif)