Highlights
Osteoarthritis is the main cause of disability; it affects approximately 10% of the world population over 60 years old.
Chondroitin sulphate could be a suitable therapeutic strategy when long term use of paracetamol or NSAID is not possible.
Its effectiveness has been widely discussed and it's funding recently questioned.
Introduction
Osteoarthritis (OA) is one of the major cause of pain and disability in the adult population1. It is a degenerative disease that affects joints and surrounding tissue of the hips, hands, knees and spine2,3. Disease progression causes gradual loss of quality of life, leading to pain, stiffness, swelling, effusion and crepitus in the affected joints4.
In Spain, according to data reported in the study “Prevalence of Rheumatic diseases in adult population in Spain” (EPISER 2016), the prevalence of OA in the population over 40 years is 13.83% (knee), 7.73% in (hand) and 5.13% (hip)1.
OA is currently a challenge for healthcare systems as its incidence is increasing due to population ageing2,4,5. The main goal of OA treatment is to reduce pain, disability and ultimately, to avoid joint replacement surgery6.
Given the safety problems associated with long-term use of acetaminophen and non-steroidal anti-inflammatory drugs (NSAIDs)2,7,8, and the characteristics of the disease and of patients with OA (generally elderly, polymedicated and with comorbidities), it seems necessary to incorporate more tolerable agents in the non-acute therapy of OA4,7.
Chondroitin sulfate (CS) is one of the main components of cartilage. CS binds to its core protein, constituting proteoglycan, which gives elastic and mechanical properties to cartilage. In addition, CS exhibits anti-inflammatory activity by inhibiting the nuclear translocation of NfkB, a protein involved in various chronic inflammatory disorders9,10. It also plays a role in subchondral bone homeostasis by improving anabolic/catabolic balance in the cartilage extracellular matrix11by decreasing the catabolic activity of chondrocytes through inhibition of proteolytic enzymes such as collagenase, elastase, phospholipase or N-acetylglucosaminidase and by enhancing the synthesis of proteoglycans (in vitro)and endogenous hyaluronic acid (in vivo)12,13.
Chondroprotection is the most recognized action of CS10. Unlike NSAIDs and acetaminophen, several studies have shown that CS has a structural modifying effect by slowing the rate of cartilage loss. Chondroprotection remains after treatment discontinuation due to a carry-over effect3,6,12,14.
CS efficacy has been widely discussed and its public financing by the Spanish National Health System has been questioned2,15).The aim of this paper was to perform a narrative review of the literature published in the last 5 years regarding the clinical and economics aspects of CS in the management of OA.
Methods
A literature search was performed until March 9th, 2021. Consulted databases were MEDLINE and Scopus. Search strategy keywords were “osteoarthritis”, “chondroitin sulfate”, “treatment outcomes”. The clinical trials inclusion criteria were as follows: randomized double-blind controlled trials focused on CS clinical outcomes in OA patients that include pain, function or radiological tests as co-primary results with a follow-up time ≥4 months and drop-outs documentation, studies published between January 2016 and January 2022. The exclusion criteria were studies of CS administered other than orally or in combinations with other drugs, sample size <50, in vitro studies or animal experimentation. Articles that studied combinations of CS with glucosamine or other chondroprotectors were not included. In the case of studies that investigated the cost-effectiveness of CS in OA treatment, no restrictions were applied by publication period. No language restrictions were applied. Titles, abstracts and full text of the articles were screened by two reviewers. When necessary, disagreements were resolved by a third reviewer.
Results
Clinical aspects of CS in the management of OA
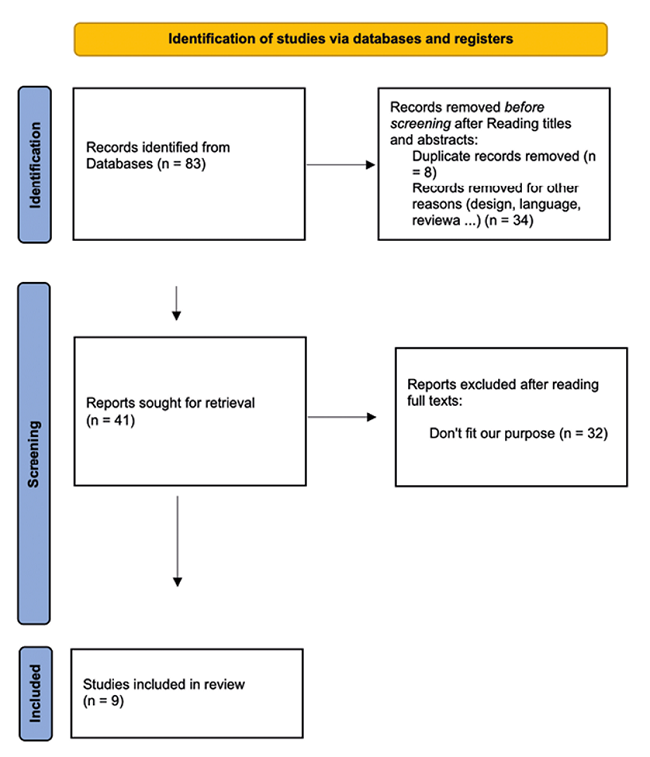
A total of 83 articles were identified with the initial search. After reading titles and abstracts, 42 articles were excluded after reading titles and abstracts and 41 were selected for full-text review. Finally, 9 articles that met the established selection criteria were included in this work to review recent evidence on the clinical potential of CS in the consulted databases.
Regarding the design of the clinical studies included, Randomized Clinical Trials (RCT) predominated6,10,11,14, sample size was between 64-604 patients. Follow-up time was highly variable: 2414, 1211, 6,6and 4 months10.
Studies that evaluated joint function coincided in using Lequesne Index (LI)6,11, In turn, all the studies that assessed pain evolution used the Visual Analogue Scale (VAS)6,11. Magnetic Resonance Imaging (MRI) for quantification of the evolution in the joint space14,16,17or functional Magnetic Resonance Imaging (fMRI)10for the assessment of the painful reaction after pressure stimulation, were also applied (Table 1).
Table 1: Clinical aspects of CS in OA management
| Author, year, (reference) | Study type | Patients, n | Follow-up (months) | Study endpoints | Outcome measurement method | Comparison group | Main findings |
|---|---|---|---|---|---|---|---|
| Pelletier et al,14, 2016 | Multicenter, randomized, double-blind, double dummy | 194 | 24 | CVL in the femoral condyle and tibial plateau of the knee | MRI | Celecoxib | Structural modification in the medial condyle with CS at 24 months. Comparable effects for pain and function between CS and NSAID (p=0,008) |
| Monfort10, 2017 | Randomized, double blind, placebo controlled | 64 | 4 | Brain response to painful pressure on patellofemoral cartilage | fMRI | Placebo | CS-treated group reflected a significant reduction in brain response activation in the primary somatosensory cortex p<0.05 |
| Raynauld17, 2017 | Post hoc, double-blind, double-dummy, comparative phase III | 120 | 24 | Evolution in knee bone curvature | MRI | Celecoxib | CS protected cartilage better than celecoxib in the condyle, and medial plateau (P≤ 0.030) |
| Reginster et al.6, 2017 | Multicenter, randomized, double blind | 604 | 6 | Pain Function | VAS IL | Celecoxib Placebo | Both CS and celecoxib showed a significant improvement in VAS (p=0.001 for CS and p=0.009 for celecoxib) and LI (p=0.023 for CS and p=0.015 for celecoxib) compared to placebo |
| Morita11, 2018 | Multicenter, Randomized, Double Blind, Dose Comparison | 73 | 12 | Pain Function | VAS IL | CS at low doses | Statistically significant difference in VAS between CS low-dose and high-dose group was observed in patients with severe baseline symptoms (LI ≥8), (p <0,05) |
CS: Chondroitin Sulfate; CVL: Cartilage Volume Loss; fMRI: functional Magnetic Resonance Imaging; LI: Lequesne Index; MRI: Magnetic Resonance Imaging; NSAID: Non-Steroidal Anti-Inflammatory Drug; OA: Osteoarthritis; VAS: Visual Analogue Scale
Pelletier J-P, et al14demonstrated by MRI in patients with knee OA, the structural modifying capacity of long-term CS in slowing down cartilage volume loss (CVL) versus celecoxib. Results revealed a slower disease progression in the CS-treated group compared to the celecoxib-treated group, showing a lower CVL from the first year of treatment. Significant differences were obtained with the celecoxib group after 24 months in the compartment (p=0.018) and medial condyle (p=0.008) for the group treated with CS and both groups experienced clinical improvement quantified on the WOMAC scale (Western Ontario and McMaster Universities Osteoarthritis).
Monfort et al.10conducted a randomized, double-blind, placebo-controlled trial in which 22 patients received 800 mg of CS and 27 received placebo daily. Two fMRI tests were performed at each session by applying painful pressure on the knee interlining and patellar surface. Main outcome was attenuation of the response evoked by painful stimulation of the knee in the brain, and was assessed at baseline and after 4 months of treatment. fMRI showed a significant reduction in the mesencephalic periaqueductal gray region with CS (p<0.05). Pre/post-treatment comparison confirms the direction of this effect, showing reduced activation only in the CS group (p<0.05). The CS-treated group reflected a significant reduction of activation in the primary somatosensory cortex (including cortical representation of the leg) (p<0.05).
Raynauld et al.17)carried out a post hoc study from the Pelletier et al. clinical trial comparing 57 patients on CS treatment with 63 on celecoxib. They investigated regions of baseline bone curvature that were best associated with reduced CVL and examined whether the change in bone curvature in these regions after 2 years of treatment is correlated with the protective effect of CS. The bone curvature regions of interest, including the medial posterior condyle and lateral central condyle, were found to correlate best with medial condyle CVL at 2 years (r=0.33, p=0.008). MRI measurements were performed at baseline and after 2 years of treatment to assess bone curvature and CVL. In patients with more flattened bones, CS demonstrated a protective effect on CVL compared to celecoxib in the medial compartment (p=0.037). Furthermore, CS protected cartilage better than celecoxib in the condyle, and medial plateau (P≤ 0.030).
The CONCEPT study6also shown the chondroprotective properties of CS, being superior to placebo and comparable to celecoxib in evaluations of knee function by LI and pain by VAS in the management of knee OA. In addition, it was the first trial that followed the recommendations of the European Medicines Agency (EMA) in an attempt to standardize protocols 18incorporating a placebo group and a control group treated with celecoxib as a requirement of external validity, given the inconsistency of previous studies. All patients were included according to the American College of Rheumatology criteria. Both CS and celecoxib showed a significant improvement in VAS (p=0.001 for CS and p=0.009 for celecoxib) and LI (p=0.023 for CS and p=0.015 for celecoxib) compared to placebo at 6 months.
Finally, Morita et al.11compared different doses of CS in patients with knee OA and grade 2-3 pain according to the Kellgren-Lawrence (KL) score. Patients randomly received 260 mg/day (low-dose) or 1560 mg/day (high dose) of CS. Joint function (LI) and pain (VAS) were assessed every 3 months. The high-dose group had better results in pain reduction, especially in patients with a higher baseline KL score. Symptom-relieving effect was not statistically significant in global analysis. However, in the subgroup analyses of patients with severe baseline symptoms (LI ≥8), high-dose of CS showed superior efficacy on reducing pain compared with low-dose CS (p <0,05).
Economic aspects
In Spain, Rubio-Terrés et al.8conducted a 6-month cost-minimization prospective study in 530 OA patients treated with NSAIDs or CS to evaluate the cost-effectiveness of both therapeutic strategies and rescue analgesia dependence. The primary endpoint was mean cost per patient. CS prescription was found to have a notable budgetary impact for the National Health System, along with a reduction in NSAID consumption (monotherapy or in combination with CS). Semiannual cost per patient with CS was 141 € versus 182 € with NSAIDs monotherapy. Savings of more than 38.7 million € over 6 months were estimated. This study has limitations such as the assumption of equal efficacy between the compared treatments or the short period of evaluation of CS (a slow-acting drug).
Discussion
OA is a degenerative disease that causes a gradual loss of quality of life2,4. CS appears to be a suitable strategy for the long-term management of OA, as it exhibits both anti-inflammatory and chondroprotective activity as well as less adverse effects than conventional treatment2,6,9. However, its effectiveness has been extensively discussed3,6,10,11,16,17and its funding by the Spanish national health system has been questioned2,15.
SYSADOA (slow-acting symptomatic osteoarthritis drugs), including CS, are justified by their structural modifying effect by slowing CVL. Despite controversies about its efficacy, CS is widely used in many countries, both medical prescriptions or over the counter. In turn, CS stands out for presenting no differences in terms of safety compared to placebo in RCTs20. The combination of relative efficacy with low risk of adverse effects has contributed to its popularity as an over-the-counter supplement7.
Wandel et al.21, in a meta-analysis of 10 RCTs with a minimum of 200 patients as inclusion criteria, reported in 2010 that neither CS nor its combination with glucosamine showed efficacy in pain reduction and in the evolution of minimum joint space width in hip or knee OA. In 10-cm VAS, mean difference in pain intensity compared with placebo was -0.3 cm for CS. Differences on minimum joint space width were also minimal compared to placebo. However, two meta-analyses from 2018 agreed on the positive effects of CS: Simental-Mendía M et al.22found a significant reduction in pain estimated by VAS for CS over placebo in patients with knee OA (p<0.00001). On the other hand, Zhu X et al.23)concluded that CS improved pain-related symptoms (p=0.003) and joint function (p=0.002) versus placebo.
However, high design heterogeneity of the included studies, as well as variability in the quality of the active ingredient CS (obtained from natural sources), could have contributed to the lack of consistency observed and to the generalization of results.
Clinical trials that evaluated CS clinical response in patients with OA presented certain limitations: a) relatively small sample sizes11,14,16,17, b) high variability with respect to disease severity at baseline, contributing to the underestimation of the effects of CS14. c) Other limitations in the design such as lacking placebo comparison (even though placebo effect is well recognized in patients with OA)14. d) Use of CS of variable quality (nutraceutical or pharmaceutical)10, which hinders the generalization or comparison of the findings of each study. Differences in product quality may contribute to the existing uncertainty regarding CS biological effects on cartilage and its clinical efficacy6,13,24,25. In turn, the proinflammatory effects of CS observed in some studies could be due to the presence of contaminants in the formulation, derived from production and purification processes or from its own origin24,26, e) Finally, there is a high level of subjectivity in the instruments used to evaluate the response to chondroprotective therapy (questionnaires and pain scales)1,7,12,13. Clinical trials identified in the last 5 years provide innovative methods aimed at objectifying response assessment. Measurements in bone curvature17, serum biomarkers16,27,28or painful response by MRI10,16,17have proven to be useful novel methods.
Martel-Pelletier et al.16conducted a post hoc Pelletier et al. study to explore serum biomarkers that could be associated with improved outcomes of CS therapy. CVL was assessed by MRI and 8 biomarkers were selected at baseline: Hyaluronic acid (HA), C reactive protein (CRP), adipsin, leptin, N-terminal propeptide of collagen IIα (PIIANP), C-terminal crosslinked telopeptide of type I collagen (CTX-I), matrix metalloproteinase-1 (MMP-1) and MMP-3. Patients were treated with 1200 mg of CS (n=57) or 200 mg of celecoxib (n=62) in a 2-year randomized controlled trial. Patients treated with CS showed lower CVL in the medial compartment, condyle and plateau (p≤0.047). Moreover, CS-treated patients with higher metalloproteinase values had lower CVL in the medial compartment, condyle and plateau (p ≤ 0.050). This study suggested a new approach to the analysis and interpretation of interindividual variability in biomarker levels and introduced the concept of metabolic responders for CS-treated patients. CS showed a potentially greater response on CVL in knee OA patients with lower inflammation and higher cartilage catabolism.
On the other hand, MRI showed the therapeutic potential of CS to reduce VCL and to preserve joint space, which is necessary to improve the clinical symptoms of OA by reducing joint pain and stiffness6,14. It should be noted that the delay of joint replacement surgeries is the main goal of chondroprotective treatment17.
Regarding the pharmacoeconomic aspects and the expected decrease in rescue analgesia derived from the chondroprotective effect attributed to CS, Lagnaoui et al.19carried out a cross-sectional observational study in 199 randomly selected French pharmacies reported an association between NSAID usage and the length of CS treatment. A marked reduction in NSAID use was observed in patients with long-term CS. Long-term users of CS had significantly lower current (44.4 versus 52.5%, p<0.05) and long-term use of NSAIDs (11.8% versus 18.5%, p<0.05)19. This study showed that real-life long-term use of CS may be associated with a lower use of NSAIDs. This could be interpreted as an indication of efficiency since it decreases the risks and costs associated with NSAIDs use.
However, this assumption is contradicted by a recent study. Barraquer et al.29, conducted a 6-month retrospective observational study involving 354 patients with OA, with the aim of assessing whether the deprescription of SYSADOA (Symptomatic Slow Action Drugs for Osteoarthritis) leads to a symptomatic worsening of OA and to an increase in analgesic consumption. A comparison was made of the consumption of analgesic drugs and NSAIDs in the 6-month period prior to withdrawal of SYSADOA versus consumption in the 6 months following withdrawal. No statistically significant differences (p>0.05) were found in the consumption of total analgesics pre/post SYSADOA withdrawal (3.97 vs 4.04 packs respectively). This study shows that discontinuation of SYSADOA does not lead to an increase in analgesic consumption, suggesting that it does not lead to OA worsening and that the economic burden associated with the prescription of these drugs should be allocated to other services or technologies supported by stronger evidence.
In none of the studies that used an active comparator, NSAIDs6,14,16,17, CS did not show inferiority in the observed results. Moreover, CS, like SYSADOA, given their suggested structural modifying effect, are characterized by a slow onset of action in the first six weeks of treatment, but also by a carry-over effect lasting up to even two months after use7,30.
In the updated algorithm for the management of knee osteoarthritis from the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO)4. However, previous works such as the systematic review and meta-analysis by Wandel et al.21did not find significant differences with respect to placebo and invite health authorities to discourage financing SYSADOA. The trend observed in recent studies on the evidence of CS in the management of OA was the incorporation of innovative outcome measures to objectify the clinical response, in the face of the well-known placebo effect in this type of study, however, despite their results being significantly favorable front, they have not been able to recover the confidence that was deposited in them by the health systems.
Epidemiological data1highlight the need to minimize the economic and health impact attributed to OA, a disease with an upward trend due to increased life expectancy. For this reason, the approach of more efficient and safe pharmacological therapies acquires special interest, given the characteristics of the patients to whom OA treatments are frequently directed, elderly with previous comorbidities who in turn require other pharmacological treatments.
In this sense, current guidelines on the management of hip and knee OA, do not compare the safety of treatment modalities. Aweid et al.31, in their systematic review on the safety of different OA treatments, highlighted the high rates of gastrointestinal and renal complications or cardiovascular risk due to treatment with diclofenac, ibuprofen or celecoxib, respectively. In contrast, only rare adverse reactions that generally do not require discontinuation of treatment or very rare (edema or allergic reactions) have been observed in CS treatment9. Therefore, the deprescription of CS could represent an irreplaceable therapeutic gap for the preserving of quality of life in certain patients who require a therapeutic alternative if NSAIDS are not tolerated.
The strategy proposed with the treatment with CS could also adequately respond to the chronic and degenerative nature of the disease, by inducing structural changes in the cartilage that could delay its loss. In addition, the carry-over effect after weeks of treatment3,6,12,14represents another added value in the necessary long-term management that deserves to be more extensively studied.
Studies of cost-minimization or reduction of rescue analgesic consumption(8,19, 29)are still scarce, and perhaps they did not sufficiently consider the disparity in the mechanisms of action of the compared pharmacological alternatives. Well, traditional management with NSAIDs and paracetamol, provide symptomatic improvement for short periods but without effects on the progression of the disease. It is possible that in order to make visible the real benefits of CS, studies better focused on long-term management are required. On the other hand, CS and glucosamine have different mechanisms of action, their combined treatment could potentially be more effective than the individual use of each drug32.
It is necessary to continue verifying if the defunding of the CS really represents a lost opportunity or if its financing is an extra cost for the health systems. For this purpose, the ESCEO recommends limiting studies on the management of OA with CS to those that exclusively use CS of pharmaceutical quality4. More evidence is needed to predict whether the expected savings from defunding CS (along with the other SYSADOAs) could be reversed by increased sick leave, hospitalizations, or surgeries in the future.
Conclusions
The set of clinical studies reviewed provide consistent evidence on the therapeutic potential of CS in OA management. New studies that objectify the clinical response of CS in the management of OA with new outcome measures could continue to contribute to clarifying their evidence.
CS may address the need for safer long-term treatments focused on the chronic nature of OA and delaying tissue degeneration which causes disability, pain and rescue analgesic dependency that provides only short-term symptomatic relief.
CS could be a cost-effective pharmacological strategy, minimizing NSAID consumption. However, this evidence is scant. More studies are required to elucidate CS economic profitability.