Highlights
This research proposes the development and use of a serum enriched with probiotics for those skin conditions that are due to a lack of skin hydration.
Probiotics when administered orally or topically in sufficient and adequate quantities, confer health benefits, in an unknown way, to their host.
It has been developed a moisturising serum with Lactobacillus fermentum CECT 5716, with acceptable characteristics in terms of organoleptic characteristics, pH, conductivity, viscosity and stability.
Introduction
Probiotics, understood as commensal microorganisms that usually form part of our microbiota, when administered, orally or topically, in sufficient and adequate quantities, confer health benefits, in an unknown way, to their host. This mechanism is hypothesised to modulate innate and adaptative immune systems by either indirect or direct pathways, which triggers antiinflammatory effects by stimulating Treg cells, the release of antiinflammatory cytokines (such as IL-10), the reduction of proinflammatory cytokines, the modulation of growth factors’ production, the competition with pathogens for nutrients and adherence to the skin surface, the coating of pathogens with immunoglobulins, the inhibition of toxins’ production by other bacteria, the activation of the production of antimicrobial peptides by epidermal keratinocytes, the improvement of wound healing, antioxidants’ production beneficial in reducing UV damage, and promotion of skin hydration and dermal thickening1,2,3,4,5,6,7,8. All the mentioned activities play an important role in the proliferation and differentiation of skin keratinocytes, which are essential for skin barrier repair, as well as in supporting the skin’s immune activity in critical skin conditions9.
In order to preserve a satisfactory water content (20-35 %) in the stratum corneum, a soft skin and a positive impact on the skin microbial community, all moisturizers should act through 4 basic mechanisms (occlusion, moisturisation, generation of hydrophilic matrices and photoprotection)6,7,10,11,12) and can contain different actives, such as antioxidants, bioactive compounds, pigments, or probiotics, among others1. However, there are several drawbacks related to topical application of probiotics, such as harsh environmental skin conditions and environmental exposure to the body, continued association with clothing, hygiene, formulations’ composition or controversial regulatory issues regarding their approval, reasons why many products include lysates or ferments instead of live microorganisms13. Despite this, in cases where these have been overcome and live probiotics have been used, taking into account that the acceptable limit for total non-pathogenic microorganisms in cosmetic products for facial application is currently set at 1000 colony forming units/gram (CFU/g) or mL (CFU/mL), the results have proven extremely beneficial13,14,15,16.
Topical probiotics, like skin microbiota, are extremely diverse, and so, depending on the genus and species of probiotics employed, their effects are different, ranging from the stimulation of the production of β-defensins by the genus Lactobacillus, to the prevention of infections and tissue regeneration by Lactobacillus species (L.plantarum, L.casei, L.brevis), the increase of hyaluronic acid synthesis by the genus Bifidobacterium or a specific strain of Streptococcus thermophiles as well as the improvement of skin collagen structuring and antioxidant activity17. By virtue of the mentioned facts, probiotics are being assayed as treatments for various skin conditions as skin ageing, reactive or sensitive skin or dry skin, among others, obtaining encouraging results. Even, there are several clinical trials running which are testing the effectiveness of different probiotics in skin pathologies such as atopic dermatitis, psoriasis or rosacea, and some marketed products including probiotics per se18.
As evidenced in several researches, probiotics have shown a beneficial effect in the treatment of skin conditions either by topical application or by ingesting alive microorganisms14. However, there are multiple criteria to take into account for its selection, which includes its safety for the host and the environment; its ability to survive on the skin surface, as in this case, where probiotics are intended for topical application9,14,19; as well as their acceptable concentration20,21) and marketing16,20. Thus, due to the efficacy of serums formulations as carriers of active agents, this research proposes the development and use of a serum enriched with probiotics for those skin conditions that are due to a lack of skin hydration.
Materials and methods
Materials
The formulation of the moisturising facial serum specifically designed for the subsequent incorporation of probiotics contains the following active ingredients13,21,22: argan oil (INCI: Argania Spinosa Kernel Oil)23, soybean oil (INCI: Glycine Soja Oil)24) and rosehip oil (INCI: Rosa Canina Fruit Oil)25) supplied by Guinama (Valencia, Spain); hydroxypropylcellulose (INCI: Hydroxypropylcellulose)26,27) supplied by SigmaAldrich; corn starch (INCI: Zea Mays Starch)28, sodium hyaluronate (INCI: Sodium Hyaluronate)29,30) supplied by Fagron (Barcelona, Spain); probiotics Lactobacillus fermentum CECT 5716 using a marketed product (Lactanza®).
The reference serum, with regenerative and rejuvenating properties, in addition to hyaluronic acid, argan oil and rosehip oil, contains also grape seed oil (INCI: Vitis Vinifera Seed Oil )31, pomegranate seed oil (INCI: Punica Granatum Seed Oil)32, supplied by Guinama (Valencia, Spain); and, growth factors33,34.
Methods
Formulation development
The moisturising serum was developed by the gradual addition, under mechanical agitation, of all the raw materials of the aqueous phase in a container, with the same process being carried out with the raw materials of the oily phase. The order of addition of the raw materials was determined at the product formulation stage. The emulsion of the serum was made at 70ºC by adding the oil phase to the aqueous phase under constant mechanical agitation. After emulsification, once a temperature of 25°C has been reached, volatile compounds such as fragrances had been added. The serum was kept in hermetically sealed containers until it was used for the studies to be carried out, its subsequent application and, after adding the probiotics, the determination of probiotic viability.
Characterisation of serums
Physical analysis of organoleptic properties, to define their particular properties, such as colour, odour, visual viscosity, as well as any other aspect of interest.
pH study, an essential parameter in all cases involving human consumption. For pH determination, the Crison-2001 micro pH-meter with the semi-solid electrode was used. Three pH measurements were taken for each serum after correct calibration of the pH-meter.
Viscosity study. For measurements was used the rheometer Thermo Scientific™ HAAKE RotoVisco 1 rheometer with coaxial cylinders of the appropriate size35. The outer, larger diameter, coaxial cylinder, is filled with enough of the serum samples to be tested, into the centre of which the smaller diameter rotating coaxial cylinder is inserted. The concrete selected working method includes a ramp up, a constant speed rotation and finally a ramp down. From this experiment are defined shear rates and flow curves with speed ramps with automatic temperature programmes (20 ºC) recorded by the HAAKE RheoWin Job Manager and HAAKE RheoWin Data Manager software36. As with the pH, 3 measurements were taken for each serum sample.
Conductimetry study, in which Crison EC Meter Basic 30+ conductivity meter37) was used. The importance of this measurement lies in the potential of the serums to allow electrical current to flow through them based on the molecules of which they are composed.
Determination of the water content of formulations
Its main role, aside from measuring the water content, is to determine the accelerated stability of the product under extreme conditions38. For this purpose, a certain amount of serum was weighed in pre-weighed beakers and then placed in ovens at 60 ºC and 40 ºC, being the water content of the samples equal to the difference between the initial weighting and the final weighting, although intermediate weightings were carried out. The study ended when the appearance and weights of the serums did not vary significantly.
Determination of stability of the designed formulation
To determine the preliminary stability of the formulation, the possible changes in its organoleptic properties as well as its resistance to contamination, it was kept in an uncovered container, at slightly different ambient temperatures and exposed to natural and artificial lighting until no further visual changes in its appearance were observed.
Determination of consumers’ perception of the product’s efficacy
To evaluate the efficacy of the facial moisturising serum, samples of the formulation were offered to 11 Caucasian volunteers (10 women and 1 man) of different age ranges (20 years, 40 years and 60 years) for continuous application onto the face’s skin once a day for at least 7 days, having obtained previously their consent and participation acceptance. At the end of the testing period, these volunteers were encouraged to complete another questionnaire, in which both positive and negative effects derived from the application of the moisturising serum were evaluated.
Determination of the viability of probiotic
Determination of the viability of probiotic strains in the moisturising serum, which was carried out by means of a plate culture method using a selective medium such as M.R.S. Agar. For this, it was necessary to prepare Petri dishes with M.R.S. Agar (CM0361) and buffered Peptone Water (CM0509) according to the indications supplied by Oxoid. Both were prepared under sterile conditions using a VAPOUR-Linelite autoclave. The M.R.S. Agar has been spread in Petri dishes in a Class 100 Flow Cabinet and stored cold until use, while the Peptone Water has been stored at room temperature in closed containers until use.
The probiotic strain used was Lactobacillus fermentum CECT 5716 as an innovation in this area and hypothesising good results based on its beneficial activity on the inflammatory condition of mastitis39, which was added to the moisturising serum prior this study, as preliminary research.
For the calculation of the viability of the probiotics was used the equation:
Serial dilutions were carried out with the aim of seeding in Petri dishes using a Class 100 Flow Cabinet, in triplicate, 3 different concentrations of probiotics (3 × 103 CFU/mL, 3 × 102 CFU/mL, 3 × 10 CFU/mL) as a positive control. After incubation in an oven at 37 °C for 24h in a hermetically sealed container and a Thermo Scientific “AnaeroGenTM 2.5 L Atmosphere Generation Systems” anaerobiosis sachet, CFU plate count was performed.
To determine the viability of the probiotics in the moisturising serum, a concentration of 105 CFU/mL was added to 25 mL of the serum as a preliminary study. After 30 min after mixing the probiotics with the moisturising serum, 5 mL of the serum was taken and made up to a final volume of 30mL with Peptone Water, and then serial dilutions were made by seeding two dilutions in triplicate (1.6 × 104 CFU/mL or 1.6 × 102 CFU/mL, and 1.6 × 103 CFU/mL). The same procedure was repeated after 1 h, 2 h, 3 h, 24 h, 7 days and 14 days. After incubation in the same conditions as the positive control, the CFU count was carried out on a plate. Finally, the viability of the probiotics in the moisturising serum was calculated.
Results and discussion
Characterisation of serums
Physical analysis of organoleptic properties
As for the moisturising serum, it is opaque white, with a characteristic odour of the added essences and a low viscous appearance. On the other hand, the regenerating and rejuvenating serum has a more yellowish but opaque colour, a characteristic odour and is slightly more viscous than the moisturising serum. All of the above is justified by the intrinsic and individual characteristics of the raw materials included in their formulations.
pH study
The average of the 3 measurements for the moisturising serum is 4.35 ± 0.025 while for the regenerating serum it is 5.9 ± 0.015. Given that facial skin accepts pH values in the range of 4 to 6, both serums would be suitable as it has been confirmed that their application does not have negative or adverse effects on facial skin, with the moisturising serum being in the lower pH range while the regenerating serum is in the upper range. In the case of the moisturising serum, due to the proposed application, it is particularly important to control these values as the viability of the probiotics could be affected by this parameter. However, in this case, these values are considered adequate since the strain used does not present strict pH ranges40,41,42.
Viscosity study
The data provided by the programs have been represented in Fig.1 and Fig.2. Fig.1 shows that the traces do not correspond to straight lines, denoting proportionality between strain rate and stress, so Newtonian behaviour of both formulations is ruled out. Since the rheograms start from the origin, both serums flow from the beginning, however, as the sliding force (rotational speed) increases, the slope (viscosity) of the curve decreases. Since all these characteristics correspond to pseudoplastic fluids, it is appropriate to state that both serums fall into this type of fluid, while ruling out the thixotropic behaviour as upward and downward ramps curves practically coincide43,44.
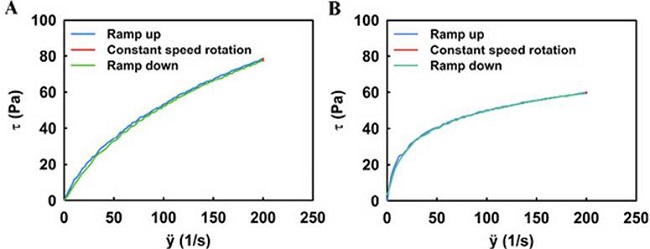
Figure 1. Representation of the effort (t) made, in Pa, by the smaller coaxial cylinder when rotating inside the larger cylinder, as a function of speed (ÿ), in 1/s, at each moment of the study for the moisturising serum (A) and the regenerating and rejuvenating serum (B).

Figure 2. Representation of the viscosity (η), in Pa*s, versus the rotational speed of the coaxial cylinder of smaller diameter (ϔ), in 1/s of the first replica of the moisturising serum (A) and regenerator/rejuvenator serum (B) differentiating between ramp-up (1), constant speed rotation (2) and ramp-down (3) at 20ºC.
Fig.2shows that the regenerative serum has a higher initial viscosity than the moisturising serum. However, the capacity of the regenerating serum to decrease its viscosity more rapidly when the rotation of the cylinder is faster than in the case of the moisturising serum is noteworthy. To find approximate viscosity values, a multitude of laws and models have been developed. In the case of pseudoplastic fluids, Power’s law stands out, but it has the limitation that it can only be applied in cases of relatively small strain rates, which is why this model does not apply in the case we are dealing with44,45.
Another suitable method for pseudoplastic fluids is the Herschel-Bulkley model, expressed through the equation:
where σ is the shear stress, σγ is the yield stress, K is an index of consistency, γ is the shear rate, and n is an index of fluid behaviour.
When n < 1, it denotes a pseudoplastic Herschel-Bulkley fluid whose viscosity decreases as the strain rate increases, which is already confirmed by the figures shown above44. Firstly, the values of n and K confirms the pseudoplastic behaviour of both serums. Secondly, the difference between the two serums in terms of the σ parameter is striking, with the regenerating serum having, on average, a higher value. However, the three replicates of the regenerating serum show closer σ values, while in the case of the moisturising serum the variation is greater. This phenomenon can be explained by the molecular interaction at the microscopic level of the various molecules present in its composition. It should be noted that the moisturising serum, unlike the regenerating serum, has a greater number of raw materials that can affect the fluctuation of its viscosity in such short times subjected to various deformation forces44.
Conductimetry study
In pharmacology or cosmetics, electrical conductivity is measured to determine the purity of water or the type of microemulsion manufactured, differentiating between oil in water (O/W) or water in oil (W/O) emulsions, together with the structure and size of the droplets formed45,46. Since electrical conductivity is based on the ion content and transport of a solution, conductivity increases as the concentration of ions dissolved in the solution increases. Thus, the mean conductivity for the moisturising serum is 4.59 mS/cm ± 0.006, while for the regenerating and rejuvenating serum it is 4.55 mS/cm ± 0.046; so, it can be said that the conductivity of the serums is very low despite all the ingredients included, since the percentage of water in the formulations exceeds 50 %. From this point of view, it is confirmed that the serums are safe for topical application. As for the determination of the type of emulsion, for the application we are currently dealing with, it is not of major relevance given that this information is known based on the manufacture of the serums, being both O/W emulsions.
Determination of the water content of formulations
As can be seen in Fig. 3, even after a short time (1.5 h) in the ovens at both temperatures, a slight change in the colour of the moisturising serum begins to be noticed. Then, on one hand, the serums kept in the 40 ºC oven after 3 h present the same appearance as after 1.5 h; the same happens with the appearance at 23 h and at the end of the time in the oven, where, both serums present a brown colouring, more intense in the case of the moisturising serum. On the other hand, in the serums kept in the oven at 60 ºC, after 3 h in the oven, the yellowish/brownish colouring becomes slightly more intense with respect to the previous time in the case of the moisturising serum, varying very little in the case of the regenerating serum. Finally, while the colouring of the moisturising serum becomes dark brown from 23 h onwards, presenting an oily surface layer; the colouring of the regenerating serum darkens slightly, giving a dry appearance from 23 h onwards.
As seen in Fig.4, at 40 ºC, the weight loss of both serums up to 4h is approximately the same; however, from that moment onwards, the regenerating serum begins to lose more weight than the moisturising serum. At 60 °C, the regenerating serum, has a more abrupt water loss in the first 4 h while the moisturising serum, in the same period, shows a slightly less abrupt evaporation of water. After 4 h, the loss of water is of equal magnitude in both serums. Those results can be explained by the proportion of raw materials included in the formulations. Thus, the reason why the moisturising serum acquires an intense brown colour at the end of the study is because there is no antioxidant compound in its composition, unlike the regenerating serum, which does include antioxidants per se. In addition, the amount of water and oil also influences the appearance of the serums about their dryness, and the oxidation of the fatty acids with regard to their colouring. Hence, it is explainable that the moisturising serum has an oily surface layer with a dark colouring as water represents approximately 55 % of the formulation and oils approximately 20 %, in comparison to the regenerating serum which has approximately 78 % and 12 % of water and oils respectively.
Determination of stability and efficacy of the designed formulation
Determination of stability
The sample of moisturising serum exposed to ambient conditions in the cold zone showed no changes after 12 h (Fig.5). On the other hand, the samples exposed to environmental conditions in the warm zone and in the humid zone showed similar behaviour up to 24 h, with the only difference being a much oily surface appearance in the sample from the warm zone. After 6 days, the changes observed were a smooth and oily surface in the sample from the warm zone, while the sample from the humid zone presented a smooth surface but with more dry and slightly oxidised areas. These results allow us to determine the characteristics that the final dosage containers in which the moisturising serum would be stored should have. Therefore, to prevent the formulation from solar radiation, its consequent oxidation, and possible contamination, the container should be as opaque as possible, or topaz-coloured, to prevent light from passing through. As for temperature, warm and humid temperatures have been shown to affect the appearance of the serum, so keeping it in a cool, dry place would be ideal.
Determination of consumers’ perception of the product’s efficacy
A pilot test was carried out to check the effectiveness of the serum before adding probiotics. For this reason, the sample size is small and it has not been compared with a control formulation.
The efficacy of the moisturising serum was tested by giving samples to 11 volunteers of different age ranges (20-40 years, 40-60 years, >60 years). Based on the responses to the specific questionnaire elaborated, the skin types on which the serum was tested varied, so the efficacy will be determined more accurately. The application time of the moisturising serum varies between 1-3 weeks. After this time, the volunteers reported favourable results in 10 cases, providing hydration, smoothness and radiance, reducing oiliness and comedogenics, without any adverse effects. However, in a 66 years old woman, the serum has generated minor adverse effects (itching, stinging or redness) that disappeared with continued use of the product. This effect may be due to the application of high concentrations of active ingredients on very dry skin. Thus, in general terms, it can be said that the results obtained after application of the moisturising serum are favourable, fulfilling its function adequately. Nevertheless, to ensure this it is necessary to carry out a randomized study, with a control group and a greater number of volunteers.
Determination of the viability of probiotic strains in the moisturising serum
The results of the determination of the viability of the probiotics in the positive control were adequate; however, a decrease in viability over time can be observed on samples, which is represented graphically in Fig. 6.

Figure 6. Determination of the viability of Lactobacillus fermentum CECT5716 in the moisturising serum at different times.
However, by analysing the graph in depth, several sections can be distinguished, recalling bacterial growth curves47,48. In this case, the latency phase is not visible as no seeding and counting of CFU at time 0 has been carried out, although its existence is assumed because the probiotics used are freeze-dried, so their activation must be gradual, followed by bacterial cell growth without division. Next, a small logarithmic phase is identified, where bacterial growth is prominent; however, after 24 h a slight drop in viability can be distinguished. This drop may have been caused by unfavourable environmental conditions for probiotics, a factor that negatively affects bacterial growth, which tends to be maximal when conditions are ideal. After the reduction in viability after 24 h, a slow growth phase appears, which tends to stabilise up to 1 week. This phase could be identified with the stationary phase of bacterial growth, where the bacteria can no longer grow exponentially for various reasons, such as accumulation of their own metabolites that can become toxic for the bacteria themselves in high concentrations, or depletion of essential nutrients, bearing in mind that the probiotics used are anaerobic. Finally, there is a death phase where the viability of the probiotics begins to decline to very low levels. Evidently, these viability data are not the most appropriate for the application we are dealing with, so it would be necessary to investigate the reason why the probiotics have such a short survival rate in the formulation of interest; to carry out other studies with this same probiotic strain by encapsulating it, using vials with 2 separate compartments; testing with some other strain of this same probiotic, or with any other probiotic species mentioned in this work.
Conclusions
Cosmetic products include probiotics in moisturisers or in products for more severe skin conditions. In view of the need of a moisturiser for xerosis with high efficacy, it has been developed a moisturising serum with Lactobacillus fermentum CECT 5716, with acceptable properties in terms of organoleptic characteristics, pH, conductivity, viscosity and stability in normal environmental conditions. The efficacy of the serum has been demonstrated by its beneficial effects on healthy volunteers before adding probiotics. The use of L. fermentum CECT 5716 as a probiotic for this formulation could have made it an extremely beneficial topical treatment for xerosis. However, the viability of this probiotic strain must be improved so that the designed product can be viable. In conclusion, it would be interesting to have a cosmetic formulation enriched with probiotics focused on skin care to prevent and/or treat xerosis, thus avoiding skin pathologies resulting from a lack of hydration and skin care. And although the elaborated serum has been a success, it is necessary to improve the viability of the probiotic in the product.


















