Highlights
The clinical pharmacist is recognized as an essential member of the multiprofessional care team in ICU. Their performance is associated with better clinical outcomes. In Brazil, studies about pharmaceutical interventions (PI) are scarce and do not present a standard on how to classify PIs, which makes it difficult to compare the results.
This article shows the PIs carried out in two ICUs of a university hospital, using the classification proposed by Sabater et al., as well as investigating the possible factors associated with this acceptability.
A real association is established between the accepted PIs and with 1) admissions to the clinical ICU, 2) prescriptions for high-vigilance medications, 3) substitution interventions for one or more medications, and 4) therapeutic class therapeutic class blood substitutes and perfusion solutions.
Introduction
The pharmacotherapy of patients in Intensive Care Units (ICU) is complex and represents a major risk factor for the occurrence of drug-related problems and adverse events.1,2 Patients with severe clinical status and multiple drugs’ use - often as off-label or considered potentially dangerous - complicate the management of pharmacological therapies, which reinforces the importance of intensive care pharmacists for monitoring these patients.3
The intensive care pharmacists are recognized by the Society of Critical Care Medicine (SCCM) as an essential members of the multidisciplinary ICU care team, and their role is also covered by current Brazilian legislation.4-5 The impact of their participation in the intensive care team has been increasingly studied in the past years and has been associated with better clinical outcomes, reduced mortality and length of hospital stay, better infection control, promotion of adequate use of protocols for gastric ulcer prophylaxis and ICU sedation, decreased incidence of drug side effects and reduction of drug costs.6-9
The activities of intensive care pharmacists in the daily care of patients in ICUs include participation in daily clinical visits, analysis of the prescribed pharmacotherapy, drug reconciliation, identification, and prevention of drug adverse reactions and, whenever appropriate, proposal of pharmaceutical interventions (PI).10,11 PIs are defined as the professional, planned and documented action performed by a pharmacist, with the purpose of optimizing pharmacotherapies and promoting health.12
According to Shulman et al. (2015), in a study carried out in the United Kingdom, about one in six drug prescriptions in ICUs require PIs, with two-thirds of them being classified as of having moderate to high impact.13 In another study conducted in the United States of America, the presence of the pharmacist at clinical visits reduced adverse event rates by 66 % when 99 % of pharmaceutical therapy management interventions were accepted, and this had an estimated cost reduction of 270 thousand dollars a year.14
Despite there are several studies worldwide on the role of clinical pharmacists in ICUs, studies in Brazil are still scarce and do not present standardization on how to classify PIs, which hampers proper comparison of findings. Therefore, this study aimed to evaluate PIs performed in two ICUs of a university hospital, using the classification proposed by Sabater et al.15, and assess possible factors associated with their acceptance.
Methods
This was a descriptive and cross-sectional study, with a quantitative approach, which analyzed PIs directed to the multidisciplinary team in clinical and surgical ICUs of a university hospital in Fortaleza, Brazil, from January to December 2019. The study was carried out with the approval of the Research Ethics Committee of the institution, with exemption from the application of the Free and Informed Consent Form, number 2,084,853 and CAAE: 74283417.4.0000.5045.
The study site is integrated into the Unified Health System (SUS) of Brazil and offers high-complexity health care. The ICUs studied have eight active beds each for the care of adult patients, one for surgical patients, and have a multidisciplinary team structured of physicians, nurses, nursing technicians, physiotherapists, pharmacists, and nutritionists, as well as resident professionals from each area. The team of clinical pharmacists in the ICU is composed of seven pharmacists, of which one is an intensive care staff and six are resident pharmacists in intensive care. In each ICU, at least two resident pharmacists work under supervision of staff, with an average of 5 hours of care per day on weekdays.
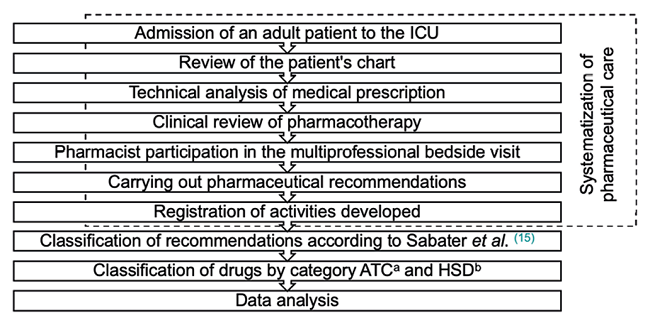
The organization of pharmaceutical care was based on 1) review of the patient’s chart with cranio-caudal assessment of clinical and laboratory parameters, 2) technical analysis of the prescription, 3) clinical review of pharmacotherapy, with analysis of need, effectiveness, safety, convenience for each prescribed drug, 4) active participation of the pharmacist in the multiprofessional bedside visit, 5) elaboration of PIs of clinical relevance and 6) recording activities developed in an internal form of the clinical pharmacy service.
Patients aging 18 years or older, admitted on any day of the week and regardless of the length of stay in the ICUs, were included. The variables collected included gender and age of patients, ICU unit type, PIs made (accepted and not accepted) and drugs involved, acceptance of interventions and reasons for non-acceptance. PIs with incomplete registration of the analyzed variables or whose acceptance was unknown were excluded. Data were collected using a standardized form at the institution. Unaccepted recommendations were also evaluated regarding the reason for non-acceptance in the following categories: 1) lack of justification, 2) hospital discharge or transfer within 24 hours after the recommendation, 3) verbally accepted, but did not change the prescription, and 4) prescriber judged the previous option as better.
PIs were analyzed for the categories of drug quantity and pharmacological strategy, using the classification proposed by Sabater et al.15 In the quantity of drug category, interventions were classified as 1) to change the dose (adjustment of the quantity of drug being administered right away) and 2) to change the dosing (change of frequency and/or duration of treatment). In the pharmacological strategy category, they were classified as 1) to add one or more drugs (addition of a new drug that was not in use by the patient), 2) to withdraw one or more drugs (abandon of the administration of a specific drug(s) among those used by the patient) and 3) to replace one or more drugs (replacement of any drugs among those used by the patient by others with different composition or of different pharmaceutical form or administration route).
Drugs involved in the recommendations were classified by the second level of the Anatomical Therapeutic Chemical (ATC) and by the categorization of High Surveillance Medicines (MAV), established by the Institute for the Practice of Safe Medication (ISMP) - a non-governmental, independent, non-profit organization that works to promote safe practices in the use of medicines and health products in Brazil.16,17 The age variable was categorized into the elderly and non-elderly groups, considering that, in Brazil, elderly are individuals aging 60 years or older.18
The data were collected and analyzed by pharmacists using Microsoft Excel®, version 2016. Numerical variables were presented as mean and standard deviation and categorical variables were exposed as frequency to investigate risk factors associated with acceptance of PIs. A significance level of 5 % was adopted. When investigating association between variables, Fisher’s exact test was performed, due to the small sample size, in the Graph Pad Prism® statistical program, version 7.0d (USA). A scheme of the study’s methodological flow is shown in Figure 1.
Results
In this study, 305 patients were included, most of them male (55.1 %; n= 168), elderly (52.8 %, n= 161) and treated in the surgical ICU (51.4 %, n= 157). The mean age of the patients was 57.9 (SD: 15.9).
A total of 1,333 PIs were made in both ICUs, of which 16 (1.2 %) were excluded due to lack of information needed for analysis. Thus, 1,317 interventions were analyzed, generating an average of 4.31 recommendations per patient. Most interventions were related to male patients (54 %; n=711) and clinical ICU patients (69.5 %, n=915). The most prevalent PIs were to replace one or more drugs (28.0 %, n=396), to add one or more drugs (27.7 %, n=365) and to change the dose (24.8 %, n=327) (Table 1).
Table 1. Demographic data and pharmaceutical interventions associated with the acceptance of the study carried out with the pharmaceutical interventions performed in the Intensive Care Units (ICU) of a university hospital in Fortaleza, Brazil.
| Variables (n=1317) | Accepted IP (n=1159) | No accepted IP (n=158) | p | RR | IC |
|---|---|---|---|---|---|
| Gender | |||||
| Female (n=606) | 534 | 72 | 0.932 | 1.002 | 0.963-1.043 |
| Male (n=711) | 625 | 86 | |||
| Age | |||||
| Elderly (n=738) | 654 | 84 | 0.443 | 1.016 | 0.976-1.058 |
| No elderly (579) | 505 | 74 | |||
| Intensive Care Unit (UCI) | |||||
| Clinical ICU (n=915) | 847 | 68 | <0.0001 | 1.193 | 1.128-1.261 |
| Post-surgical ICU (n=402) | 312 | 90 | |||
| High-Surveillance Drugs | |||||
| Yes (n=286) | 267 | 19 | 0.001 | 1.079 | 1.038-1.122 |
| No (n=1031) | 892 | 139 | |||
| Pharmaceutical interventions (PI) | |||||
| To replace one or more drugs (n=369) | 339 | 30 | 0.006 | 1.062 | 1.021-1.105 |
| To add one or more drugs (n=365) | 311 | 54 | 0.058 | 0.956 | 0.912-1.004 |
| To change the dose (n=327) | 284 | 43 | 0.492 | 0.982 | 0.937-1.031 |
| To withdraw one or more drugs (n=224) | 194 | 30 | 0.498 | 0.980 | 0.928-1.037 |
| To change the dosing (n=32) | 31 | 1 | 0.166 | 1.104 | 1.034-1.178 |
| Therapeutic classes (with more than 5 % frequency) | |||||
| Antiinfectives for systemic use (n=317) | 279 | 38 | 0.492 | 0.983 | 0.934-1.034 |
| Blood substitutes and perfusion solutions (n=144) | 136 | 8 | 0.019 | 1.076 | 1.025-1.130 |
| Drugs for acid related disorders (n=102) | 86 | 16 | 0.127 | 0.941 | 0.862-1.027 |
| Vitamins (n=84) | 72 | 12 | 0.355 | 0.960 | 0.876-1.051 |
| Ophthalmologicals (n=80) | 73 | 7 | 0.577 | 1.029 | 0.957-1.106 |
| Stomatological preparations (n=79) | 71 | 8 | 1.000 | 1.011 | 0.935-1.094 |
Regarding acceptance, 88.0 % (n=1159) of the recommendations were accepted. The recommendations that showed the highest prevalence of acceptance were to replace one or more drugs (29.2 %) and to add one or more drugs (26.8 %, n=311), followed by to change the dose (24.5 %, n= 284). Additionally, it was observed that 73.1 % (n= 843) of the accepted PIs were performed in the clinical ICU and that 57 % (n= 90) of the unaccepted ones were performed in the surgical ICU (Table 1). Overall, the most prevalent reason associated with non-acceptance (68.4 %, n= 108) was ‘they thought the previous option was better’; this reason is also the most prevalent in each ICU individually. Other reasons included lack of justification (20.9 %, n=33) and verbal acceptance of the PI, but lack of prescription change (10.8 %, n=17).
A total of 150 different drugs were involved in the PIs, the most prevalent being anti-infectives for systemic use (24.1 %, n=317), blood substitutes and perfusion solutions (10.9 %, n=144) and drugs for acid related disorders, according to the ATC classification. A higher proportion of acceptance of the interventions was observed for the therapeutic classes: systemic corticosteroids (96.9 %; n=31/32), blood substitutes and perfusion solutions (94.4 %; n=136/144) and ophthalmologicals (91.3 %; n=73/80) (Table 1).
HSDs involvement was identified in 286 (21.7 %) of the PIs, which occurred mainly in the clinical ICU (74.5 %; n=213). Overall acceptance was 93.4 % (n=267). The main PIs involving HSD were to add one or more drugs (n=102), to replace one or more drugs (n=92) and to change the dose (n=55). The most prevalent therapeutic classes were blood substitutes and perfusion solutions (48.6 %, n=139), followed by antithrombotic agents (16.8 %, n=48), being the most frequent drugs potassium chloride, (n=47), magnesium sulfate (n=33) and unfractionated heparin (n=31) (Table 2).
Table 2. Main therapeutic classes and drugs involved by type of pharmaceutical interventions (PI) in the study performed in the Intensive Care Units (ICU) of a university hospital in Fortaleza, Brazil.
| PI n (%) | Therapeutic class | n (%) | Most frequent drugs |
|---|---|---|---|
| To replace one or more drugs 369 (28.0 %) | Blood substitutes and perfusion solutions | 52 (14.1 %) | Potassium chloride (n=17), Magnesium sulfate (n=14) |
| Antiinfectives for systemic use | 48 (13.0 %) | Vancomycin (n=10), Piperacillin/Tazobactam (n=7) | |
| Vitamins | 40 (10.8 %) | Thiamine (n=16), Vitamin K (n=12) | |
| Others | 229 | - | |
| To add one or more drugs 365 (27.7 %) | Blood substitutes and perfusion solutions | 63 (17.3 %) | Potassium chloride (n=22), Magnesium sulfate (n=13) |
| Antiinfectives for systemic use | 50 (13.7 %) | Vancomycin (n=13), Piperacillin/Tazobactam (n=12) | |
| Stomatological preparations | 42 (11.5 %) | Chlorhexidine 0.12 % (n=42) | |
| Others | 210 | - | |
| To change the dose 327 (27.8 %) | Antiinfectives for systemic use | 164 (50.1 %) | Meropenem (n=41), Piperacillin/Tazobactam (n=24), Vancomycin (n=24) |
| Blood substitutes and perfusion solutions | 19 (5.81 %) | Potassium chloride (n=6), Magnesium sulfate (n=5) | |
| Drugs for acid related disorders | 18 (5.5 %) | Omeprazole (n=15), Ranitidine (n=3) | |
| Others | 126 | - | |
| To withdraw one or more drugs 224 (17.0 %) | Drugs for acid related disorders | 62 (27.7 %) | Omeprazole (n=56), Ranitidine (n=6) |
| Antiinfectives for systemic use | 43 (19.2 %) | Vancomycin (n=8), Meropenem (n=8) | |
| Analgesics | 13 (5.8 %) | Tramadol (n=3), Metamizole (n=3), Morphine (n=3), Acetaminophen (n=3) | |
| Others | 106 | - | |
| To change the dosing 32 (2.4 %) | Antiinfectives for systemic use | 12 (37.5 %) | Amikacin (n=4) |
| Vitamins | 5 (15.6 %) | Vitamin K (n=2), Vitamin C (n=2) | |
| Calcium channel blockers | 3 (9.4 %) | Amlodipine (n=3) | |
| Others | 12 | - |
The associations of accepted and non-accepted PIs with the variables gender, elderly and non-elderly categories, inpatient units, PPM prescription, type of PI and therapeutic classes are described in Table 1. The Fischer test showed an association between the acceptance of the interventions when performed in the clinical ICUs (p<0.0001, as opposed to surgical ICUs) and in the presence of HSD (p=0.0013). In addition, significant associations were observed between acceptance of PIs and to replace one or more drugs PI (p=0.0062) and the class blood substitutes and perfusion solutions (p=0.0187). Regarding other variables, there was no other statistically significant association (Table 1).
Discussion
This is the first study that evaluates PIs in ICU environments in Fortaleza using the classification proposed by Sabater et al. and evaluating possible factors associated with PIs acceptance.15 PI were performed by intensive care pharmacists as a strategy for optimizing pharmacotherapy and preventing adverse events, highlighting their important role in reviewing medical prescriptions. In addition, PIs acceptance was associated with clinical ICU admissions, HSD prescriptions, to replace one or more drugs interventions and the therapeutic class blood substitutes and perfusion solutions.
The benefit of the pharmacist’s involvement in the care of critically ill patients in this study can be observed by the large number of PIs (n=1317) and their high acceptance rate (88.0 %). Further, in comparison with the study performance by Fidelis et al. which explored PIs between 2010 and 2013 in the same ICU evaluated in the present study, it is noted an increase in the number of PIs and acceptance rates.11 This finding is an indicator of improved quality of clinical pharmacy service and corroborates the role of the clinical pharmacist is well established in the ICUs of the hospital. Other studies report acceptance rates ranging from 64 % to 99.6 %.14,19-22 Factors such as prioritization of high-risk PIs, effective communication between different healthcare professionals and availability and openness to discussions from physicians may explain these acceptance rates.
Most of the PIs were performed for patients hospitalized in the clinical ICU (69.5 %), and a statistically significant association was found between clinical ICUs and accepted interventions, in as opposed to post-operative ICUs. Similar findings were reported in the study from Maciel et al.19 In fact, patients admitted to clinical ICUs use higher number of drugs, especially antimicrobials, have longer hospital stays and more clinical complications, when compared to patients admitted to the post-operative ICUs, thus being more susceptible to prescription errors and consequently to PIs. Furthermore, the clinical ICU has intensive care medical residents and a fixed daily bedside clinical visit time, which increase the possibility for discussions within the healthcare group and creation of bonds of trust between the pharmacist and the multidisciplinary care team.
The most prevalent PIs in this study were to replace one or more drugs (28 %) and to add one or more drugs (27.7 %), both considered as pharmacological strategies. Despite the difficulty in comparing studies due to the different classifications of PI, Reis et al. and Maciel et al. also reported prevalence rates of 18.9 % and 16.5 %, respectively, of these PIs.19,21 On the other hand, Bourne et al. reported higher prevalence rates of interventions related to add one or more drugs (28.2 %) and dose revision (25.8 %), while Fideles et al., point out a predominance of the PI of dilution management (14.4 %) and dose adjustment (12.0 %).11,23 These variations in the prevalence rates of types of PI can be influenced by the patient population, clinical pathologies, professionals involved, maturity of the clinical pharmacy service and level of integration of the multidisciplinary ICU team.11
A statistically significant association between the pharmacological strategy to replace one or more drugs and acceptance was observed (p=0.0062), being 29.2 % of the overall accepted PIs. On the other hand, there was a slight tendency of non-acceptance of PIs such as adding drug. These results indicate the need to assess the reasons associated with medical non-acceptance of certain types of PIs and to build and disseminate protocols at an institutional level that can lead to standardization of care and alignment of approaches.
Antiinfectives for systemic use formed the main class of drugs targeted by PIs (24.1 %), with the intervention ‘Dose modification’ (51.7 %) being the most frequent PIs for this class, and meropenem (18.9 %) and vancomycin (17.7 %) the main representatives. Similar results were described in the studies from Sereno et al. (33 %) and Fidelis et al. (52.7 %).11,24 In clinical practice, it is observed that antimicrobial doses are extremely important in the management of infections in critically ill patients, especially in cases of sepsis and septic shock. Moreover, other factors, such as obesity, positive fluid balance, renal and/or hepatic failure are quite common and require frequent dosage adjustment, with daily monitoring, to avoid clinical worsening, emergence of toxicity or increased microbial resistance.25
Errors in the use of systemic antimicrobial drugs have potential implications for both the individual and the population. The proper use of antimicrobial agents and their deprescription, when needed, with continuous application of pharmacokinetic principles and evaluation of bacterial resistance are key.26 After initiation of antimicrobial therapy, daily assessment of the need, effectiveness and safety of the antimicrobials prescribed by a clinical pharmacist can reduce the risk of toxicity and guide actions to prevent and control bacterial resistance. In this context, the importance of implementing Antimicrobial Stewardship programs with the active participation of a clinical pharmacist is emphasized.27
In this study, the involvement of HSDs was identified in 21.7 % of the PIs, showing a high acceptance rate of 93.4 %. The two HSDs most involved in PIs were potassium chloride and magnesium sulfate, both representing the therapeutic class blood substitutes and perfusion solutions, which were also associated with greater acceptance. Similar results were found in a prospective study carried out in Spain by Miarons et al.28 In critically ill patients, electrolyte depletion is common, and a usual goal is to correct them to achieve normal serum values (mainly potassium and magnesium) and reduce the risk of ventricular tachycardia and other arrhythmias.29 HSDs have a greater risk of causing more serious damage to patients if used incorrectly, when compared to other medicines. Prescribing HSDs requires double-checking prescriptions prior to administration, as well as identifying patients at high risk. Therefore, these drugs are potentially subject to greater institutional surveillance, becoming targets of PIs.30
This study provides valuable information about PIs performed in two ICUs in Fortaleza, which may be useful for the methodological adequacy of similar studies. However, it has some limitations. The study was carried out in only one hospital, with few active beds, and the presence of scarce clinical data made it impossible to perform a broader assessment of the associated factors. Thus, these data cannot be extrapolated to other centers, rural hospitals, and institutions without the presence of a clinical pharmacist in the daily care of the patient in the ICU. In addition, the different classifications, and standardizations of PIs in different studies make it difficult to compare findings.
Conclusion
This study enabled the evaluation of several PIs performed in two ICUs using the PIs classification proposed by Sabater et al.20 Further, we observed high PIs acceptance rates by the medical team, suggesting that the role of the intensive care pharmacist is consolidated in the hospital of the study. The acceptance of the interventions was statistically associated with admission to a clinical ICU (vs surgical), presence of high surveillance medicines, intervention to replace one or more drugs and therapeutic class blood substitutes and perfusion solutions. Finally, this study reinforces the role of the intensive care pharmacist in the review of medical prescriptions as a strategy for optimizing pharmacotherapy and preventing adverse events. In addition, we highlight the need for the use of PIs classifications that are standardized in the literature in order to simplify comparisons between studies.