INTRODUCTION
Breast cancer is the most common cancer in women and is one of the leading causes of cancer-related deaths [1]. Breast cancer, which has a heterogeneous structure, has been molecularly subtypes according to its hormone receptor (HR) and human epidermal growth factor receptor 2 (HER2) status. Treatment approaches and survival times also vary according to the subtypes [2, 3]. One of the subtypes, HER2-positive breast cancers, is an aggressive subtype that constitutes 15-20% of breast malignancies and has a high risk of recurrence [4]. In addition to conventional chemotherapies, HER2-targeted therapies significantly improved treatment outcomes of HER2-positive breast cancer patients [5].
In this study, we aimed to investigate the prognostic factors affecting the response time and the effect on survival as well as drug toxicity related to T-DM1 in patients received T-DM1 treatment in our center.
MATERIAL AND METHODS
STUDY POPULATION
This study has been designed as a single center and retrospective study. The institutional ethics committee approved this study (approval no: 2022.121.06.11). The study included outpatient metastatic breast cancer patients who received treatment between 2016 and 2022. The study included patients with: 1) HER2 positive breast cancer with pathological diagnosis; 2) 18 years of age or older; 3) patients who completed at least 2 months of T-DM1 therapy; 4) metastasis was confirmed in organs using computed tomography, magnetic resonance imaging scans, or other imaging methods; 5) concomitant or no previous history of malignancy; 6) no active infectious disease, no immunosuppressive drug use. All patients received at least one line of cytotoxic chemotherapy (taxane) and trastuzumab for metastatic disease and progressed on or after the last treatment.
The HER2-positive disease was considered as those with positive results by immunohistochemistry (IHC) 3+ or IHC 2+ expression and those with positive results by fluorescent in-situ hybridization (FISH). According to the guide of the American Society of Clinical Oncology/College of American Pathologists, those with estrogen receptor (ER) and progesterone receptor (PgR) above 1% were evaluated as positive [11].
TREATMENT
Patients were treated with T-DM1 at a standard dose of 3.6 mg per kg of body weight intravenously every 21 days. Computed tomography (CT) and positron emission tomography (PET-CT) was used at 3-4 cycle intervals to evaluate the treatment response. Treatment responses were determined according to RECIST (Response Evaluation Criteria In Solid Tumors) 1.1. According to the information obtained from the patient archive files, adverse events were defined according to the Common Terminology Criteria for Adverse Events v5.0 (CTCAE).
STATISTICAL ANALYSIS
Progression-free survival (PFS) was defined as the time from the beginning of the T-DM1 treatment to the time of any documented clinical progression, relapse, or death from any cause. Overall survival (OS) was defined as the time from the diagnosis to death for any reason. All statistical analyses were performed using SPSS version 26.0 (IBM Corb, Armonk, NY). Survival analysis was performed using the Kaplan-Meier method and the Log-Rank test was used for group comparison. Univariate vs multivariate analyses of factors affecting survival were created with the Cox Proportional-Hazards Model. Statistical significance defined as a P value<0.05.
RESULTS
56 patients were included in the study. All of them consisted of female patients and the median age was 56 (range 33-88). While there was progression after T-DM1 treatment in 39 patients (69.6%), 30 patients (53.6%) were dead at study completion. The number of HR negative (ER and PgR negative) patients was 23 (41.1%). 13 patients (23.2%) had brain metastases, 24 patients (42.9%) had liver metastases, and 39 patients (69.6%) had bone metastases (Table 1).
The best responses of patients to T-DM1 treatment were detected as complete response (CR) in 5 patients (8.9%), partial response (PR) in 30 patients (53.6%), stable response (SR) in 10 patients (19.6%), and progression (PD) in 11 patients (19.6%). Objective response rate (CR; complete plus partial response) was 62.5%. Median best response time was 4.1 months (95% CI 4–7.1).
Table 1. Demographics, tumor, and clinical characteristics of patients

BMI: Body Mass Index; ECOG: Eastern Cooperative Oncology Group; ER: Estrogenic receptor; PgR: Progesterone receptor; CNS: Central nervous system; T-DM1: Trastuzumab emtansine; Her2: Human epidermal growth factor receptor 2.
SURVIVAL TIMES
The median follow-up period in our study was 21.5 months (95% CI, 17.1-25.9). Median PFS (mPFS) for T-DM1 was 10.4 months (95% CI, 8.6-16.4) and the median OS was (mOS) 22 months (95% CI, 14.9-29.2) (Figure 1). In the created univariate cox regression model, liver metastasis (HR=2.12, 95% CI: 1.12-3.99, p=0.021), ECOG performance status (HR=3.05, 95% CI: 1.47-6.33, p=0.003), and serum CA 15-3 (HR= 2.40, 95% CI: 1.06-5.43, p=0.035) were found to be factors associated with RFS. Liver metastasis (HR=2.54, 95% CI: 1.17-5.51, p=0.019), ECOG performance status (HR=4.66, 95% CI: 1.80-12.04, p=0.002), and serum CA 15-3 (HR= 2.55, 95% CI: 1.09-6.25, p=0.041) remained statistically significant for PFS in the established multivariate analysis (Table 2). The corresponding mPFS values according to liver metastasis, ECOG performance status, and CA 15-3 were 14.5 (95% CI 11.7–17.3) versus 7.7 months (95% CI 2.7–12.7) (log rank p =0.017), 21.1 (95% CI 14.4–34.4) versus 9 months (95% CI 5.6–12.4) (log rank p =0.002), and 15.9 (95% CI 14–17.8) versus 8.9 months (95% CI 6.2–11.6) (log rank p =0.029), respectively, with statistically significant difference (Figure 2).

Figure 1. Kaplan–Meier survival curves for the study population.a: Progression-free survival; b: Overall survival.

Figure 2. Kaplan Meier survival curves for progression-free survival according to liver metastasis.(a), pre-treatment serum CA 15-3 levels (b), and ECOG PS (c).CA 15-3: Cancer antigen 15-3; ECOG PS: Eastern Cooperative Oncology Group performance status.
Table 2. Univariate and multivariate analysis for progression-free survival (PFS)
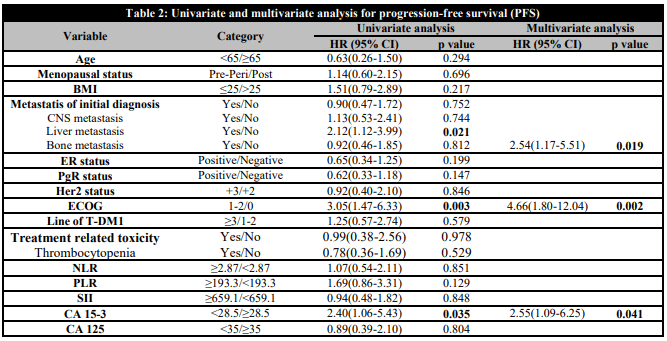
Significant values are indicated in bold.BMI: Body Mass Index; ECOG: Eastern Cooperative Oncology Group; ER: Estrogenic receptor; PgR: Progesterone receptor; CNS: Central nervous system; T-DM1: Trastuzumab emtansine; Her2: Human epidermal growth factor receptor 2; NLR: Neutrophil-to-lymphocyte ratio; PLR: Platelet to lymphocyte ratio; SII: Systemic immune inflammatory index; CA 15-3: Cancer antigen 15-3; CA 125: Cancer antigen 125.
In the univariate cox regression analysis for OS, only ECOG performance status (HR=2.61, 95% CI: 1.14-5.96, p=0.023) was found to be prognostic (Table 3). The mOS of patients with poor performance status was 18.4 months (95% CI 11.8–24.9), while mOS was 44.3 months (95% CI 16.7–71.9) in patients with good performance status.
Table 3. Univariate analysis for overall survival (OS)

Significant values are indicated in bold.BMI: Body Mass Index; ECOG: Eastern Cooperative Oncology Group; ER: Estrogenic receptor; PgR: Progesterone receptor; CNS: Central nervous system; T-DM1: Trastuzumab emtansine; Her2: Human epidermal growth factor receptor 2; NLR: Neutrophil-to-lymphocyte ratio; PLR: Platelet to lymphocyte ratio; SII: Systemic immune inflammatory index; CA 15-3: Cancer antigen 15-3; CA 125: Cancer antigen 125.
TOXICITY AND SIDE EFFECTS
In the adverse event assessment according to CTCAE v5.0, T-DM1 was found to be well tolerated in most patients. There was no patient who died due to toxicity or whose treatment was terminated due to toxicity. While toxicity occurred in 46 (82.1%) of the patients, grade 3-4 toxicity developed in 10 (17.9%) patients. Grade 3-4 toxicities that developed were fatigue (7.1%), anemia (3.6%), nausea (3.6%), headache (1.8%), thrombocytopenia (3.6%), and diarrhea (1.8%) (Table 4).
DISCUSSION
In this study, we aimed to investigate the survival effect of T-DM1 in patients who received trastuzumab and taxane treatment, the prognostic factors affecting response time of T-DM1 treatment, and T-DM1-related adverse events as a single-center experience with real-life data. In our study, we found that T-DM1 was safe and a tolerable medication in the majority of patients. Our survival analyses found that ECOG performance status, liver metastasis and pre-treatment serum CA 15-3 level were prognostic for T-DM1-related PFS. We found that only the ECOG performance status was prognostic for OS.
In the EMILIA study, which included 991 patients receiving trastuzumab and taxane, mPFS was reported as 9.6 months in advanced breast cancer patients [12]. In a multicenter study including 441 patients, mPFS was reported as 9 months while PFS was reported as 10 months in another study conducted in Italy [13, 14]. In our study, mPFS was found to be 10.4 months, similar to previous studies.
The relationship between performance status and survival time in breast cancer is known [15, 16]. As expected, this important connection was also demonstrated in studies that included patients using only T-DM1 [17, 18, 19]. In this study, ECOG performance status was found to be prognostic, and mPFS was detected as 9 months in patients with poor performance status and 21.1 months in patients with good performance status. Despite its low sensitivity, CA 15-3 levels are one of the most frequently used tumor markers in patient follow-up in daily oncology practice. The American Society of Clinical Oncology (ASCO) has reported CA 15-3 as a useful marker in making treatment decisions [20]. However, there is no consensus in previous studies that included breast cancer patients for its prognostic feature [21, 22, 23]. Ozyukseller et al. investigated the prognostic feature of CA 15-3 levels in patients using only T-DM1 and found the change in CA 15-3 levels during treatment as prognostic [17]. In our study, on the other hand, the relationship between pretreatment serum CA 15-3 levels and survival was investigated, and a longer survival time was found in patients with high pretreatment serum levels. CA 15-3 level was found to be an independent prognostic factor for patients using T-DM1.
The liver is one of the most common visceral organs to which advanced breast cancer metastasizes, and the presence of liver metastases has been reported as a poor prognosis in studies including all breast cancer subtypes [24, 25, 26, 27]. In previous studies that included patients using T-DM1, the prognostic feature of the presence of visceral metastases was investigated, but liver metastasis was not investigated as a subgroup. In the studies of Ozyukseller et al., Fabi et al., and Noda-Narita et al., visceral metastasis was not found to be prognostic, similar to our study [14, 17, 28]. However, liver metastasis status was also analyzed in our study and this was found to be prognostic for T-DM1 response time. To the best of our knowledge, this is the first study to detect the presence of liver metastases as prognostic for patients using T-DM1.
In our study, it was observed that T-DM1 was well tolerated and although 82.1% of the patients had adverse events, no treatment was changed due to toxicity in any of the patients. The rate of those who experienced grade 3-5 adverse events was 17.9%. This rate was 40% in the TH3RESA study, 25.7% in the KATHERINA study, and 37.5% in the KAMILIA study [7, 29, 30]. These differences in the incidence of adverse events between studies may be related to many factors, such as the median age of the patients included, performance status, treatment lines, and sites of metastasis [10, 31]. The most common serious adverse events were fatigue, nausea, thrombocytopenia, and anemia, consistent with previous studies [10, 32]. Further multicenter studies with large patient populations are needed for more generally accepted information on adverse events.
Our study has some limitations. The first of these is that the study has a single-centered and retrospective design. Second, even if the patient selection criteria were carefully chosen, various circumstances can influence laboratory markers. However, the strengths of the study are that it includes real-life data and extensive prognostic factor analysis for T-DM1.
CONCLUSIONS
T-DM1 is an important treatment agent that has shown its survival effect in patients with advanced HER2 positive breast cancer. In this study, we found that ECOG performance status, liver metastasis status, and pre-treatment serum CA 15-3 levels were prognostic factors associated with the response time of T-DM1 treatment. In addition, our results showed that T-DM1 is a safe and tolerable treatment agent.















