1. INTRODUCTION
Heart failure (HF) is a cardiovascular condition with high morbidity and mortality that conditions one of the most critical problems in public health with a high-cost burden on the health system, mainly about long-term drug treatments and frequent hospitalizations [1, 2].
Despite advances in recent decades in health promotion and prevention policies, the global incidence of cardiovascular disease remains on the rise. In addition, the lifetime risk of HF varies between racial and ethnic groups between 20% and 45% after 45 years, with an estimated 8 million cases in the United States by 2030, representing a 46% increase in prevalence [3, 4].
Among the most critical risk factors associated with HF development are coronary heart disease, hypertension, obesity, diabetes, valvular heart disease, and cigarette smoking, which are currently highly prevalent entities [5].
Heart failure has been divided into distinct phenotypes based on the presence of signs and symptoms and the measurement of left ventricular ejection fraction (LVEF); defining HF with reduced ejection fraction (HFrEF) as patients with LVEF ≤ 40%; mildly reduced (HFmrEF) (LVEF 41-49%); and preserved (HFpEF) in LVEF ≥ 50% with evidence of structural and functional cardiac abnormalities and elevation of natriuretic peptides. Among these phenotypes, reduced LVEF is the most prevalent and is currently the group with the most effective evidence-based recommendations [6, 7].
Although the prognosis of patients with HF has improved considerably with new drug therapies and devices, patients continue to have a marked reduction in their quality of life [8].
The discovery of the cardiovascular benefits of Inhibitors of the Sodium-Glucose Cotransporter Type 2 in patients with HF is a new pillar in clinical management regardless of the established phenotype. SGLT2 inhibitors have significantly influenced mortality, hospitalizations for acute decompensation, symptom improvement, and quality of life. However, no significant number of clinical studies still synthesize its use. Therefore, this review aims to describe the impact of SGLT2 inhibitors on mortality and rehospitalizations in patients with HF.
2. EPIDEMIOLOGY OF HEART FAILURE
HF correlates positively with age, being more frequent in people over 60 years of age. The prevalence of HF in the general population oscillates between 1 and 3%, while in people older than 65 years, it is between 5 and 9% [9].
HF mortality rates evidenced in observational studies are high, with records of up to 20% a year after diagnosis and 67% at five years [10, 11], with better survival among women than men [12].
Patients with HF are hospitalized on average at least once a year [13], and the risk of hospitalization for acute HF decompensation is 1.5 times higher in patients with pathologies such as diabetes mellitus with poor metabolic control, obesity, atrial fibrillation, and chronic kidney disease, considering these as strong predictors for HF hospitalizations [14].
3. PHARMACOLOGICAL THERAPIES IN HEART FAILURE
Pharmacotherapy, together with lifestyle change interventions, are the cornerstone of HF treatment, so these must be optimized before considering more invasive therapies with devices [15].
Patients with HFrEF have multiple evidence-based therapeutic options, affecting strong outcomes such as mortality, rehospitalizations, improvement in functional capacity, clinical status, and quality of life. Among therapeutic options stand out angiotensin-converting enzyme inhibitors (ACE Inhibitors), angiotensin receptor blockers with or without association with neprilysin inhibitors (ARB/ARNi), beta-blockers and mineralocorticoid receptor antagonists (MRA). These drugs are classified as disease modifiers, and their use is recommended in all patients with HFrEF unless there is a contraindication or there is no tolerance for them [6].
The evidence in patients with HFmrEF and HFpEF phenotypes are limited; however, the advent of SGLT2 inhibitors provides a new pharmacological strategy for managing HF, regardless of its phenotype.
4. SODIUM-GLUCOSE CO-TRANSPORTER TYPE 2 INHIBITORS
SGLT2 inhibitors are not new drugs; they initially entered the market to treat hyperglycemia in patients with type 2 diabetes mellitus. However, their beneficial effects at the cardiovascular level opened the door for new clinicians to optimize patient management with HF.
The SGLT2 cotransporter is found in the apical membrane of the S1 and S2 of the proximal convoluted tubule of the nephron, which fulfills the function of reabsorption of 90% of glucose filtered at the glomerular level. However, when its activity is inhibited, it leads to a process of glycosuria and natriuresis, which are proposed as the main properties for cardiovascular protection [16].
4.1. CARDIOVASCULAR BENEFIT
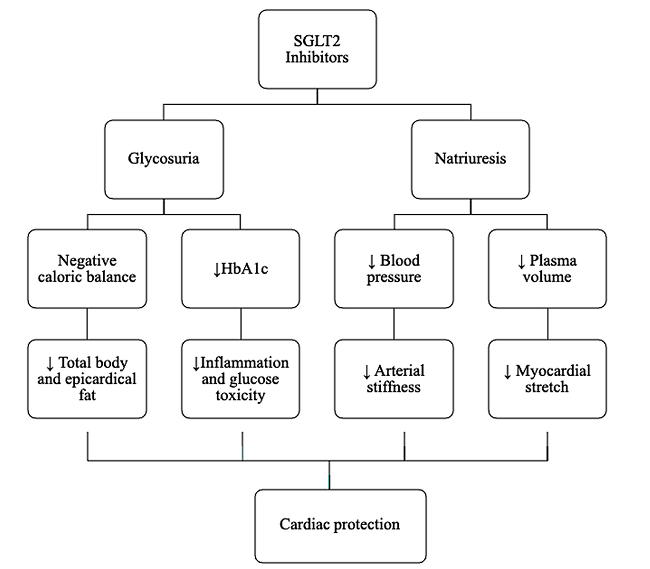
The cardiovascular benefits are summarized in (Figure 1), where glycosuria will lead to a negative caloric balance, reducing body and epicardial fat, inflammation, and glucotoxicity, and collaborating with improving cardiac contractility and mitigation of the atherosclerosis process. Additionally, natriuresis decreases plasma volume and blood pressure, leading to less arteriolar stiffness and myocardial stretching, favoring cardiovascular protection [16].
Another proposed theory about SGLT2 inhibitors is the apparent inhibition of the sodium hydrogen exchanger (NHE) at the cardiomyocyte, which activity in experimental models increases in patients with HF. NHE raises cytoplasmic concentrations of sodium and consequently calcium, which would lead to a growth in cardiomyocyte injury and the development of cardiomyopathy [17, 18].
Although more studies are needed to clarify with certainty the mechanisms of action of these drugs; some of the cardiovascular benefits are the improved control of glucose, lipids, and hypertension, decreased body mass index (BMI), reduced cardiorenal remodeling, inhibition of hormonal dysregulation, more efficient use of metabolic substrates, and inhibition of ion channels, anti-inflammatory, and antioxidant effects [19].
4.2. SGLT2 INHIBITORS IN TYPE 2 DIABETES MELLITUS
The cardiovascular benefits of SGLT2 inhibitors were discovered in response to guidance from the US Food and Drug Administration (FDA) in 2008, which required that all new hypoglycemic therapies demonstrate cardiovascular safety before being approved for the market [20].
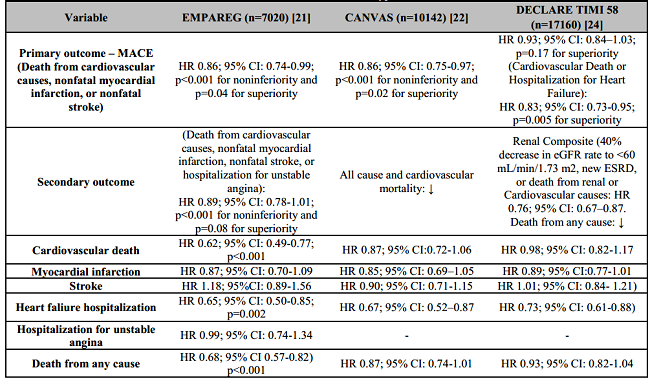
The EMPA-REG clinical trial showed that empagliflozin compared to placebo in patients with type 2 diabetes mellitus and cardiovascular disease reduced the primary outcome of major adverse cardiovascular events (MACE). It was defined by myocardial infarction, stroke and cardiovascular death in 14% [Hazard ratio (HR) 0.86; 95% confidence interval (CI), 0.74 to 0.99; p<0.001 for noninferiority and p=0.04 for superiority]. It was also oserved a lower risk of cardiovascular death by 38% (HR 0.62; 95% CI: 0.49-0.77; p<0.001) and hospitalizations for heart failure in 35% (HR= 0.65; 95% CI: 0.50-0.85; p=0.002) [21].
The CANVAS clinical trial showed that canagliflozin versus placebo in patients with type 2 diabetes mellitus and cardiovascular disease reduced the primary outcome of MACE by 14% (HR 0.86; 95% CI: 0.75-0.97; p<0.001 for non-inferiority and p=0.02 for superiority) [22]. In CANVAS, there was an increased risk of amputation of lower limbs; however this result was not evidenced in the CREDENCE trial, in which the same molecule was evaluated in renal outcomes in patients with type 2 diabetes mellitus and nephropathy, and once again was demonstrated its beneficial impact in major cardiovascular events and hospitalizations for HF [23].
The DECLARE-TIMI 58 clinical trial showed that dapagliflozin versus placebo in patients with type 2 diabetes mellitus and pre-existing or at risk of atherosclerotic cardiovascular disease (ASCVD) reduced the primary outcome of cardiovascular death and hospitalizations for HF by 17% (HR 0.83; 95% CI: 0.73-0.95; p=0.005 for superiority). It also observed a decreased HF hospitalizations by 27% (HR 0.73; 95% CI: 0.61-0.88) [24] (Table 1).
A systematic review and meta-analysis of the cardiovascular effects of SGLT2 inhibitors in patients with type 2 diabetes mellitus included pivotal studies of empagliflozin, canagliflozin, and dapagliflozin with a population of 34,222 patients. This study showed a reduction in hospitalization due to HF and cardiovascular death by 23% (HR 0.77; 95% CI: 0.71-0.84; p<0.0001) and 31% reduction in HF hospitalizations (HR 0.69; 95% CI: 0.61-0.79; p<0.0001) [25].
4.3. SGLT2 INHIBITORS IN HEART FAILURE WITH REDUCED LVEF PHENOTYPE
The SGLT2 Inhibitors cardiovascular results in patients with type 2 diabetes mellitus opened the possibility to evaluate the benefit of these drugs in patients with HF with the presence or absence of type 2 diabetes mellitus; provided by date two trials have changed the clinical practices in patients with HFrEF, which are DAPA-HF and EMPEROR Reduced [26, 27] (Table 2).
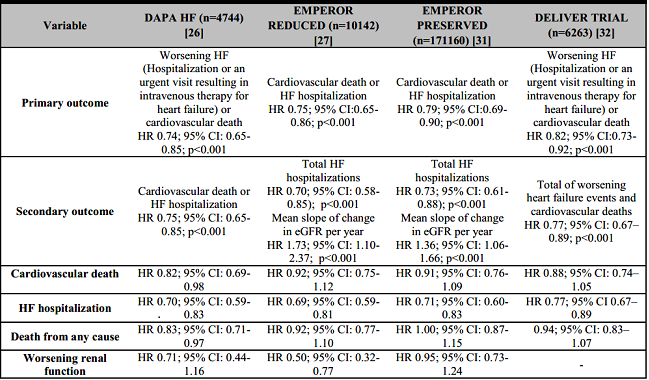
The DAPA-HF study was a placebo-controlled trial with a population of 4744 patients with HF, of whom 45% (1983) patients had type 2 diabetes mellitus as comorbidity and the inclusion was based on having a reduced LVEF phenotype with functional class II, III, or IV according to the New York Heart Association (NYHA) classification and elevated natriuretic peptides. The dapagliflozin intervention group received a 10-mg dose with a mean follow-up of 18.2 months. The impact of therapy in reducing the primary outcome of cardiovascular death or worsening of HF was 26% (HR 0,74; 95% CI: 0.65-0.85; p<0.001) and also reduced hospitalizations for HF by 30% (HR 0.70; 95% CI: 0.59-0.83), cardiovascular death in18% (HR 0.82; 95% CI: 0.69-0.98) and death of any cause in 17% (HR 0.83; 95% CI: 0.71-0.97) [26].
The EMPEROR-Reduced study was a placebo-controlled trial with a population of 3730 HF patients, of whom 49.8% (1856) patients had type 2 diabetes mellitus, and the inclusion was based on having a reduced LVEF phenotype with functional class II, III, or IV according to the NYHA. The intervention group received empagliflozin 10 mg with a mean follow-up of 16 months; with evidence of a 25% reduction in the primary outcome of cardiovascular death and hospitalizations for HF (HR 0.75; 95% CI: 0.65-0.86; p<0.001), and as a Secondary outcome reduced hospitalizations for HF by 30% (HR 0.70; 95% CI: 0.58-0.85; p<0.001) [27].
Although EMPEROR-Reduced did not show a statistically significant reduction in cardiovascular mortality, a meta-analysis of the DAPA-HF and EMPEROR-Reduced trials did show a statistically significant association in the decrease in cardiovascular mortality [28]. In addition, both therapies were associated with improvement in the physical capacity and quality of life of these patients [29, 30].
Therefore, empagliflozin and dapagliflozin reduced the risk of cardiovascular death or hospitalization for HF in patients with reduced phenotype in the presence or absence of type 2 diabetes mellitus [26, 27].
4.4. SGLT2 INHIBITORS IN HEART FAILURE WITH MIDLY REDUCED AND PRESERVED LVEF PHENOTYPE
The evidence in HF with mildly reduced and preserved LVEF phenotype has always been limited, regard to multiple clinical trials have not shown an impact in reducing morbidity and mortality. So the behaviors have been aimed at controlling comorbidities and risk factors; however, the EMPEROR Preserved and DELIVER trial recently demonstrated a new therapeutic target with solid evidence in this subgroup of patients [31, 32] (Table 2).
The EMPEROR Preserved trial was a placebo-controlled study with a population of 5,988 HF patients, 49% (2938) of patients had type 2 diabetes mellitus and the inclusion was based on mildly reduced or preserved LVEF phenotype, which 2/3 of the population had LVEF >50%. In addition, the patients must have functional class II, III, or IV according to the NYHA and elevated natriuretic peptides [31]. The intervention group in this study received empagliflozin 10 mg with a mean follow-up of 26.2 months; where the impact of therapy in reducing cardiovascular death and worsening of HF was 21% (HR 0.79; 95% CI: 0.69-0.90; p<0.001) and hospitalizations for HF were reduced by 29% (HR 0.71; 95% CI: 0.60-0.83) [31].
The DELIVER trial was a placebo-controlled study with a population of 6,263 HF patients, 55% (3457) of patients had type 2 diabetes mellitus and the inclusion was based on mildly reduced or preserved LVEF phenotype. In addition, the patients must have functional class II, III, or IV according to the NYHA and elevated natriuretic peptides [32]. The intervention group in this study received dapagliflozin 10 mg with a mean follow-up of 2.3 years; where the impact of therapy in reducing cardiovascular death and worsening of HF was 18% (HR 0.82; 95% CI: 0.73-0.92; p<0.001) and HF hospitalizations were reduced by 23% (HR 0.77; 95% CI: 0.67-0.89) [32].
4.5. SGLT2 INHIBITORS ACUTE HEART FAILURE
Acute HF can be the clinical presentation of a new onset of HF or, more frequently, worsening (Acutely decompensated chronic HF); both entities are severe with high mortality and rehospitalization rates [33].
In the SOLOIST-WHF trial, Sotagliflozin (a non-selective sodium-glucose cotransporter inhibitor) was evaluated in 1222 patients with type 2 diabetes mellitus and recent worsening of HF regardless of phenotype, where the intervention population received sotagliflozin 200 mg with a plan to increase to 400 mg according to tolerance with a mean follow-up of 9.2 months. The administration of sotagliflozin was before or maximum of three days after hospital discharge with evidence of a 33% reduction in the primary outcome of cardiovascular death, hospitalizations for HF, or visits to the emergency room that required intravenous therapies for HF management (HR 0.67; 95% CI: 0.52-0.85; p<0.001). No statistically significant differences in serious adverse events with adequate renal safety [34].
Other studies such as the EMPA-RESPONSE-AHF; was a randomized, double-blind, placebo-controlled trial that evaluated the effects of empagliflozin in 80 patients with acute decompensated chronic heart failure, defined by the presence of dyspnea with NYHA functional class III-IV, associated with clinical signs of congestion, the elevation of natriuretic peptides and under intravenous diuretic therapy. The intervention group received empagliflozin 10 mg during the first 24 hours of admission, with daily clinical evaluation from the fourth day.
Although results did not show improvement in dyspnea, NT-proBNP, response to diuretic, or length of hospital stay; this was associated with a decrease in the worsening of in-hospital HF and a reduction in the outcomes of cardiovascular death and rehospitalizations for HF at 60 days, and it was also a safe and well-tolerated drug [35]. The results of this study should be interpreted with caution, considering the limitations in the number of patients.
The recently published EMPULSE trial, which was a randomized, double-blind, placebo-controlled study that evaluated the effects of empagliflozin in 530 patients with new-onset of HF or worsening (Acutely decompensated chronic HF), regardless of LVEF. The intervention group received empagliflozin 10 mg at a mean time of 3 days from hospital admission to a follow up of 90 days; where the results showed 36% reduction in the primary outcome of cardiovascular mortality, hospitalizations for HF, and improvement in quality of life (HR 1.36; 95% CI: 1.09-1.68; p=0.0054) [36].
Empagliflozin was well tolerated, and the benefits are robust in the presence or absence of type 2 diabetes mellitus; this indicates that the initiation of empagliflozin in patients with acute HF produces a significant clinical benefit within 90 days after the start of treatment [36].
4.6. RENAL SAFETY OF SGLT2 INHIBITORS
Chronic kidney disease is another condition that limits the treatment of patients with HF, leading to a higher number of hospitalizations, mortality, and drug toxicity.
The coexistence of these two entities ranges between 40-50% [38]; however, the results in clinical trials of SGLT2 Inhibitors on renal protection confer safety in initiating this therapy.
A meta-analysis of the cardiovascular effects of empagliflozin, canagliflozin, and dapagliflozin in patients with type 2 diabetes mellitus showed a 45% decrease in progression in the renal outcome, defined as worsening renal function, end-stage renal disease (ESRD), or death due to renal cause (HR 0.55; 95% CI: 0.48-0.64; p<0.0001) [25]. Results later strengthened in two clinical trials such as CREDENCE with canagliflozin and DAPA CKD with dapagliflozin [23, 37].
In the CREDENCE trial, all patients had type 2 diabetes mellitus with glomerular filtration rates (GFR) greater than or equal to 30ml/min, while in the DAPA CKD trial, regardless of the presence or absence of type 2 diabetes mellitus, included even patients with GFR of 25ml/min.
The CREDENCE results showed a 30% decrease in the primary outcome, defined by the presence of ESRD (Need for dialysis, kidney transplant, or sustained decline in GFR below 15 mL/min), doubling of creatinine levels serum, or death due to renal or cardiovascular causes (HR 0.70; CI 95%: 0.59-0.82; p:0.00001) [23].
Subsequent DAPA CKD trial evinced a 39% reduction in the primary outcome of sustained decline in GFR ≥50%, end-stage renal disease, or death due to renal or cardiovascular causes (HR=0.61; 95% CI: 0.51-0.72; p<0.001) [37].
Trials such as EMPEROR REDUCED and EMPEROR PRESERVED included patients with GFR as low as 20 mL/min with evidence of a slower rate of GFR decline [27, 31].
Although an initial drop in glomerular filtration rate is expected in the first weeks of starting therapy with SGLT2 inhibitors, there is evidence of an upcoming stabilization. Finally, improvement over time compared to placebo [38, 39].
4.7. SECONDARY EFFECTS AND MONITORING PARAMETERS OF SGLT2 INHIBITORS
The risk of infection has been the most commonly found side effect in the aforementioned clinical trials [21, 22, 24]; mainly genital mycotic infections, however, these are usually mild with rapid resolution and a low rate of recurrence, so the measures are aimed at genital hygiene. However, in patients with severe or recurrent fungal infections, the use of SGLT2 Inhibitors should be closely monitored [40].
Urinary tract infections have also been documented with the use of SGLT2 Inhibitors, but the risk has not been increased compared to placebo in clinical trials [40].
Among the hemodynamic effects of SGLT2 inhibitors is the reduction of systolic and diastolic blood pressure (4 to 6 and 1 to 2 mmHg), respectively. In addition, an increase of urinary volume with an average of 300 ml/day is observed, which can lead to a decrease in GFR between (3–5 ml/min) during the first weeks with a subsequent stabilization, which monitoring of GFR is suggested, signs and symptoms of hypotension and volume depletion [41, 42, 43].
SGLT2 inhibitors about glucose control do not induce hypoglycemia, and considering safe drugs, however, the risk increases mainly in combination with insulins. Additionally, the risk of normoglycemic diabetic ketoacidosis is extremely rare; nevertheless, advice should be given on the signs and symptoms of ketoacidosis, and if it occurs, the medication should be discontinued and find immediate medical attention [43].
Lower extremity amputation risk was a side effect seen only with canagliflozin in the CANVAS trial [22], which FDA made a warning in 2017, and then it was rescinded in 2020 [44]. Although the association between SGLT2 Inhibitors and the risk of amputation is doubtful, Caution is suggested for those who may be at increased risk (History of prior amputation, peripheral vascular disease, severe peripheral neuropathy, diabetic foot ulcers, or infections) [45].
5. RECOMMENDATIONS IN CLINICAL PRACTICE GUIDELINOES FOR SGLT2 INHIBITORS
SGLT2 inhibitors (Empagliflozin, Canagliflozin, and Dapagliflozin) have indications approved by the FDA to reduce the risk of cardiovascular complications in patients with type 2 diabetes mellitus [46, 47, 48, 49, 50]. However, in the context of HF, the 2021 European guidelines Society of Cardiology (ESC) for the diagnosis and treatment of chronic and acute HF only recommend dapagliflozin and empagliflozin for the reduction of cardiovascular death and HF hospitalizations in patients with reduced phenotype regardless of the presence or not type 2 diabetes mellitus [6]. The recently published 2022 American College of Cardiology (ACC) heart failure guidelines positioned empagliflozin as a fundamental therapy in patients with mildly reduced and preserved HF phenotype [51].
These suggestions are based on the fact that at the time of the ESC guidelines publications there was no evidence from the EMPEROR PRESERVED and at the time of ACC guidelines there was no evidence from the DELIVER trial [31, 32].
6. DISCUSSION
Some of the benefits of SGLT2 inhibitors in heart failure are the reduction in the risk of cardiovascular mortality and hospitalizations due to acute decompensation HF despite LVEF phenotype, evinced in the DAPA HF and DELIVER trial with dapagliflozin and EMPEROR REDUCED and PRESERVED with empagliflozin [26, 27, 31, 32]. These findings remained consistent in diabetic and non-diabetic patients, showing an encouraging trend that reinforces the benefit of SGLT2 Inhibitors in all patients with HF, regardless of type 2 diabetes mellitus status [49].
These drugs during episodes of acute HF decompensation are not supported by current clinical management guidelines due to the lack of evidence supporting this therapy.
Though, the recent results of the EMPULSE clinical trial with empagliflozin in patients with acute decompensation regardless of LVEF and who were clinically stable. This term was defined as the absence of inotropic support in the last 24 hours, no increase in diuretic therapy, and absence of vasodilators in the previous 6 hours with systolic blood pressure greater than 100 mmHg in the absence of hypotension data. This study showed 36% relative risk reduction in cardiovascular mortality, hospitalizations for HF, and improvement in quality of life [36].
The findings of the EMPULSE trial align with the results of the SOLOIST-WHF trial with sotagliflozin. However, the last-mentioned was suspended early due to sponsorship conflicts, so the planned sample size was not obtained [34]. Anyhow, the EMPULSE results open the door to new clinical trials that support these findings and evaluate the possibility of starting this therapy earlier in the hospital setting.
These new medications initially introduced to the market as a treatment for type 2 diabetes mellitus, have shown a high renal safety profile [25], with low rates of hypoglycemia, and are rarely associated with normoglycemic diabetic ketoacidosis [43].
Among the most frequent adverse effects are recurrent urinary tract and genital mycotic infections [21, 22, 24]; we must choose patients according to their individual risk factors.
About the monitoring parameters, there is a reduction in systolic and diastolic blood pressure with a low risk of hypotension. Additionally, it is considered a beneficial effect in hypertensive patients [41].
The association between SGLT2 Inhibitors and the risk of amputation is doubtful; however, caution is advised in those who may be at increased risk [45].
All these findings have changed the established guidelines for the management of heart failure. Previously, the prominent representatives were ACE Inhibitors, ARB and beta-blockers, and in patients with a reduced phenotype, the addition of MRA. However, now we can see that the perspective is aimed to SGLT2 Inhibitors as a first-line drug, being cataloged as one of the "Fantastic Four" in this pathology [50]; with recommendations IA in HFrEF and IIA in HFmrEF and HFpEF according to the 2022 HF Guidelines of the ACC [51].
Some of the notable strengths of this review are the compilation of the most significant number of multicenter randomized studies of SGLT2 Inhibitors currently available on this pathology. It allows us a better understanding of the population evaluated and also stands out the adequate sample inclusion of the American population in the trials, which makes the results extrapolated to our patient population.
One of the limitations is the cost. However, that must not be a contraindication to prescribe them.
In the future, the trend with SGLT2 Inhibitors is aimed at investigating other factors that impact the quality of life of patients with HF, such as obesity, dyslipidemia, and exercise capacity, where studies are already being advanced.
7. CONCLUSION AND RECOMMENDATIONS
Up to 50% of patients with HF have T2DM and some degree of renal involvement, and the coexistence of these three entities contributes to increased morbidity and mortality.
The advent of SGLT2 Inhibitors provides a new therapeutic target with solid evidence results, mainly in decreased cardiovascular mortality and HF hospitalizations with an adequate renal safety profile, and this cardiovascular benefit has been reproduced in clinical trials regardless of the presence or absence of T2DM and the HF phenotype based on LVEF.
Despite its cardiovascular benefits, health professionals, mainly in patients with chronic kidney disease, limit its use in clinical practice. Therefore, it is suggested to create multidisciplinary teams at institutions that provide health services, and this groups must be compounded by cardiologists, nephrologists, and endocrinologists for the monitoring and evaluation of the pharmacological adherence of patients after the start of SGLT2 Inhibitors, and this way maximizes the benefits.
SGLT2 Inhibitors have been positioned as a fundamental pillar in the management of HF, indicating its onset increasingly earlier, avoiding systematic algorithms, not delaying cardiovascular protective effects, and improving patient prognosis. SGLT2 inhibitors, as a disease-modifying drug, should be prescribed in all heart failure diagnosed patients unless there is a contraindication.