Medicina Oral, Patología Oral y Cirugía Bucal (Internet)
ISSN 1698-6946
Oral biopsy in dental practice
Amparo Mota-Ramírez1, Francisco Javier Silvestre2, Juan Manuel Simó3
(1) Degree in Dental Surgery. Diploma in Hospital Clinical-Surgical Dentistry
(2) Assistant Professor of the Department of Stomatology, Valencia University Dental School, and Head of the Stomatology Unit, Dr. Peset University Hospital
(3) Degree in Medicine and Degree in Dental Surgery. Staff physician of the Stomatology Unit, Dr. Peset University Hospital. Valencia. Spain
ABSTRACT
The conclusions drawn from the study of an oral biopsy are considered essential for the definitive diagnosis of diseases of the oral mucosa, and for the subsequent planning of appropriate treatment.
Although the obtainment of biopsies is widely used in all medical fields, the practice is not so widespread in dental practice - fundamentally because of a lack of awareness of the procedure among dental professionals. In this context, it must be taken into account that the early diagnosis of invasive oral malignancy may be critical for improving the patient prognosis.
However, in some cases the results are adversely affected by incorrect manipulation of the biopsy material. The present study provides an update on the different biopsy sampling techniques and their application. Such familiarization in turn will contribute to knowledge of the material and instruments required for correct biopsy performance in dentistry, as well as of the material required for correct sample storage and transport.
Key words: Oral biopsy, oral histology, surgical technique, oral diagnosis.
RESUMEN
La utilización de la biopsia oral se considera una prueba irrefutable para el diagnóstico definitivo de enfermedades de la mucosa bucal y la subsiguiente planificación de un enfoque terapéutico adecuado.
Aunque esta técnica está ampliamente extendida en todas las especialidades médicas, en el ámbito de la odontología no está muy divulgada, fundamentalmente por el desconocimiento de la misma por parte del odontólogo general; y cabe tener en consideración que un diagnóstico temprano de un cáncer oral invasivo pueda mejorar de forma radical el pronóstico del paciente.
Sin embargo, en ocasiones el resultado se ve malogrado por un inadecuado manejo del material a analizar. El objetivo de este trabajo es actualizar las diversas técnicas para obtener la muestra y su aplicación. Nos facilitará, en gran medida, conocer el material e instrumental que precisemos para su toma en odontología y el material más apropiado para la conservación y el transporte de la muestra.
Palabras clave: Biopsia oral, histología oral, técnica quirúrgica, diagnóstico oral.
Introduction
The word biopsy originates from the Greek terms bios (life) and opsis (vision): vision of life. A biopsy consists of the obtainment of tissue from a living organism with the purpose of examining it under the microscope in order to establish a diagnosis based on the sample (1).
The technique allows us to establish the histological characteristics of suspect lesions, their differentiation, extent or spread, and to adopt an adequate treatment strategy. Biopsies establish evolutive control of disease processes, and are able to document healing or relapse. In turn, the biopsy findings are of irrefutable legal medical value (2,3).
A biopsy is indicated in application to any lip or oral mucosal lesions following the exclusion of local irritants (of traumatic or inflammatory origin), when the lesions in question are seen to persist for more than two weeks, and may be suggestive of malignancy (1,4). In general, lesions appearing in the oral mucosa should be explored and evaluated for the possible presence of local irritative factors. If such factors are identified, they must be eliminated, after which an observation period of approximately 15-20 days is indicated. After this period of time, and if the lesions persist, histopathological study is required to discard possible malignancy (3). Such a study is also indicated in the case of radiotransparent bone lesions presenting radiological features suggestive of malignancy - even when such features constitute casual findings in the course of a routine radiological study.
All maxillary cysts, and particularly keratocysts, must also be processed for histological study (1,3).
A biopsy is also indicated in the case of bone lesions accompanied by pain, sensitivity alterations or other symptoms, and in application to bone lesions showing important changes or rapid expansion as evidenced by successive radiological evaluations.
A biopsy is also required of those oral mucosal surfaces that show important and persistent color changes (becoming very white, red or pigmented) or changes in appearance (cracking, proliferation or ulceration), with deep-lying hard masses detected upon palpation.
Likewise, evaluation is required of premalignant mucosal lesions or states such as lichen planus or leukoplakia, in persistent atrophic-erosive areas (4).
A biopsy is also very useful for the detection of certain systemic illnesses requiring histological confirmation in order to establish the definitive diagnosis, e.g., lupus, amyloidosis, scleroderma, or Sjögrens syndrome - which can be confirmed by an oral tissue biopsy. As an example, confirmation of Sjögrens syndrome requires the obtainment of a sample of the lesser salivary glands of the lips (1,3,4).
A biopsy is also used as complement in the diagnosis of certain disorders of infectious origin, such as lesions suggestive of syphilis or tuberculosis, based on an oral sample - though prior confirmation of the positivity of tests specific of such disease processes is required.
Another indication for biopsy is confirmation of the diagnosis of blister lesions, in mucocutaneous diseases affecting the oral mucosa, such as vulgar pemphigus or cicatricial pemphigoid.
Benign tumors, with the exception of those of a vascular nature, are to be removed, sending the entire sample for histopathological study to determine the histological origin of the lesion, after establishing a tentative diagnosis (4).
On the other hand, a biopsy is contraindicated in very seriously ill patients, in those subjects with some systemic disorder that may worsen, or where secondary complications may develop (4).
Likewise, a biopsy should be avoided in the case of lesions located in very deep regions or in areas of difficult access where the surgical technique proves complicated or hazardous, with the risk of damage to neighboring structures. In such cases the patient should be referred to a specialist. The same considerations apply in the case of suspected vascular lesions such as hemangiomas, due to the risk of massive and persistent bleeding (1,5).
Biopsy is not advised in the case of multiple neurofibromas, due to the risk of neurosarcomatous transformation, or in tumors of the greater salivary glands. Such biopsies must be performed by specialized surgeons, in order to avoid damaging nearby anatomical structures and causing the spread of tumor cells, as this would adversely affect the prognosis (1).
In turn, a biopsy would be needless in application to banal irritative lesions or normal anatomical variants such as physiological gingival pigmentation, geographic tongue, linea alba, lingual indentations, protuberances, exostosis, etc. (3,6).
Types of biopsy
Depending on the characteristics of the target lesion, the biopsy is defined as direct (located superficially, with easy access) or indirect (when the lesion lies in depth and is covered by normally appearing mucosa or tissue)(3). However, biopsies can also be classified according to the technique used, the material employed, the clinical timing, the location of the target lesion, processing of the sample, and the purpose of the biopsy.
1) The technique employed:
Depending on the technique employed, biopsies can be classified as incisional or excisional. The incisional technique involves the removal of a representative portion of the target lesion and of a part of healthy tissue (3,7,8). If the lesion is extensive, different samples should be obtained, placing each of them in a separate and adequately identified container. Along with the report sent to the pathologist, a schematic representation of the lesions should be attached, specifying the original location of each sample (1,3,9). Such an approach is indicated in the case of suspected malignancy or precancerous lesions. Likewise, such a multiple sample biopsy should be performed when the target lesion is difficult to remove due to its large size or complicated location. It is also indicated for establishing the diagnosis of a systemic disease process.
Controversy exists as to the possibility that incisional biopsies of malignant lesions may increase the risk of metastasis, by disrupting the barrier preventing migration of the neoplastic cells and thus favoring invasion of the bloodstream at the site of the surgical wound (10).
In certain tumors such as hemangioma or melanoma, the biopsy should be performed with complete and extensive resection of the lesion, in order to avoid severe bleeding or metastatic spread, respectively (9).
An excisional biopsy in turn involves total removal of the lesion, with slight peripheral and in-depth safety margins, applicable to papillomas, fibromas or granulomas (4,11). Such biopsies play a diagnostic and therapeutic role, since complete removal of the lesion is carried out, ensuring the inclusion of a peripheral margin of normal tissue (12).
2) The material used:
A number of cutting instruments can be used when performing a biopsy: a conventional scalpel, a punch, and the so-called B-forceps. Electroscalpels and CO2 laser scalpels deserve separate mention, for although they are used by some authors, their associated inconveniences make them scantly recommendable for obtaining a biopsy sample.
The oral mucosal punch is a rapid, simple, safe and inexpensive technique for obtaining a representative sample of most oral zones. The technique and usefulness are similar to those of the skin punch. The instrument consists of a sterile and discardable punch with a plastic handpiece and cylindrical cutting blade. The latter may be 2, 3, 4, 5, or 6 to 8 mm in diameter, with stepwise increments of 0.25 a 0.50 mm. As a result, tissue cylinders 2 to 8 mm in diameter can be obtained - the most widely used caliber being 4 mm.
The punch is grasped between the index and thumb, supporting the cylinder over the target lesion. If a small-diameter cylinder is obtained, suturing of the residual wound is usually not necessary, and the bleeding can be contained by simply applying a piece of gauze or surgical dressing. The wound heals by second intention, with good esthetic results. In other cases, primary wound closure can be performed with sutures.
Punches are typically made of plastic or metal. The metal presentations can be reused, and are to be sterilized before use. In contrast, the plastic variants are less expensive, weigh less and are destined for single use (13,14).
The punch is able to obtain several samples at the same time, and at different points, and generates less patient anxiety than the conventional scalpel (15).
However, the punch is unable to remove large lesions, and cannot be used in intensely vascularized or innervated areas. It is likewise not applicable to deep lesions, and is limited to epithelial or superficial mesenchymal target tissues. Caution is moreover required when using the punch to sample lesions located over important submucosal structures such as the mental or nasopalatine foramen. On the other hand, the instrument is difficult to use in the region of the soft palate, maxillary tuberosity or floor of the mouth, due to the lack of firm tissue fixation or support, and the mobility of the target zone (13,14).
Other instruments, such as the so-called B forceps, can also be used to obtain a biopsy. This instrument was developed by Bermejo (16), and facilitates, simplifies and especially homogenizes soft tissue biopsies of the oral cavity and of the lesser salivary glands. These forceps are equipped with two cusps - one with a window - to allow compression of the target tissue between them. The target zone is positioned exposed within the window, and the compressive effect of the cusps allows us to work in an ischemic field within the window. Compression by the forceps causes the sectioned portion, freed from its peripheral connective tissue attachments, to propel from the window - thus allowing us to measure the depth of the sample, with easy access to the base in order to facilitate sectioning (16).
On the other hand, while the electroscalpel has the advantage of causing no bleeding, since it cauterizes the vessels, its main inconvenience is the induction of thermal damage. Although similar, the laser scalpel produces less extensive thermal damage and less postoperative pain. However, in the same way as the electroscalpel, it is currently not advised for obtaining oral biopsies.
3) Clinical timing of sampling:
Depending on the clinical timing of the biopsy, the procedure can be classified as intraoperative or extraoperative.
An intraoperative biopsy allows a rapid histopathological diagnosis. The sampled material is processed without fixation, frozen with dry ice. In this sense, freezing at a temperature of between -40ºC and -60ºC produces a tissue consistency that allows sectioning with the microtome. The quality of the preparation under such conditions is less ideal, the analysis is more difficult, and the pathologist faces only three diagnostic options: positive, negative or doubtful (1,3,7).
However, the result can be received in the operating room in a short period of time, thus allowing the surgeon to continue with the operation as required. This procedure is indicated when a malignant tumor is suspected, and surgery can be planned according to the histological findings of the intraoperative biopsy. However, the technique is not always reliable, and in cases of doubt surgery is postponed until conclusive results are obtained from the study of tissue embedded in paraffin.
A very important procedure is the intraoperative examination of the margins of a resected malignant tumor, to evaluate the possible existence of tumor invasion beyond these margins. In such a case, wider resection is required (12).
On the other hand, an extraoperative biopsy requires a longer processing time. The fixed tissue sample is processed and embedded in paraffin, followed by the cutting and staining of thin sections. These preparations offer greater quality than frozen samples, and histopathological evaluation is therefore easier (1,3).
4) Sampling location:
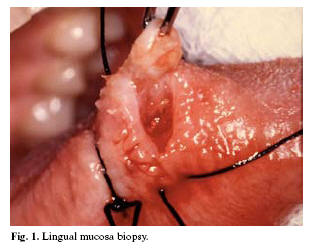
Depending on the topography involved, the biopsy can be obtained from the oral mucosa in its different locations (Figure 1), the salivary glands, bone, lymph nodes, and other head and neck tissues. A biopsy of the oral mucosa is simple to perform, and is used to distinguish among different types of lesions, in order to define adequate treatment or conduct follow-up over time. A conventional biopsy is usually indicated (6).
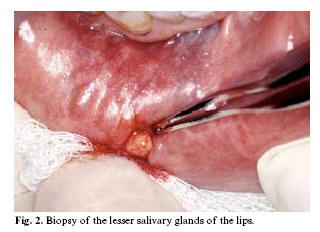
Regarding the salivary glands, it is very common and easy to obtain a biopsy of the lesser salivary glands of the lips for diagnosing or confirming an autoimmune condition such as Sjögrens syndrome (Figure 2). All the typical microscopic characteristics of this syndrome, with the exception of the clusters of myoepithelial cells, are usually observed in the lesser salivary glands, and the lip glands are the most widely used option, in view of their accessibility. Following local anesthetic infiltration of the zone, a small incision measuring approximately 10 mm is made in the mucosa of the lower lip; the thickness of the sample should be sufficient to obtain the glands without affecting the muscle layer or arteries (1). In the case of retention cysts such as lip mucoceles, an excisional biopsy is indicated (1,3).
On the other hand, when biopsying the greater salivary glands, and specifically the parotid gland, fine-needle aspiration biopsies (FNAB) are increasingly used, due to their non-invasive nature. An intraoperative biopsy is also of interest in this context, selecting the tumor target zone and avoiding nerve structures. However, greater salivary gland biopsies, and particularly of the parotid gland, are rarely performed outside the context of surgery, unless the lesion is superficial and malignancy is suspected (6).
A bone biopsy in turn constitutes an indirect technique. These procedures are usually more difficult, and require the raising of a soft tissue flap and an adequate approach to the bone layer. After raising the mucoperiosteal flap, a chisel and mallet are used, or the target zone is drilled to obtain the specimen. In this sense, mention should be made of the trephine drill, composed of a hollow cylinder with a cutting edge, that allows the harvesting of bone cylinders of different sizes. In the case of certain bone tumors, a piece of trabecular bone can be collected using a curette. Bleeding can be countered with oxidized cellulose, a gelatin sponge or bone wax, followed by suturing of the overlying mucosal layer. The specimen thus obtained is sent to the pathologist together with a detailed report on the patient history, and clinical and radiological characteristics. It is often advisable for the surgeon to see the X-rays with the pathologist and radiologist, before establishing the definitive diagnosis. In some cases, such as when specimen decalcification is required, the laboratory may need more time to obtain the results (1,3,6,17).
Lymph node biopsy is also an indirect procedure. In this context, adenopathies are commonly the result of inflammatory or neoplastic processes. Prior clinical evaluation and laboratory testing is required before biopsying lymph nodes. If a biopsy is decided, then the entire lymph node should be surgically removed.
5) Processing of the sample:
Depending on the processing procedure involved, the sample can be analyzed frozen, or embedded in paraffin or methacrylate, and can be examined under the electron microscope, or as a fresh sample. Molecular analyses are also possible.
As commented above, frozen biopsies allow a rapid histopathological diagnosis. This procedure is usually used during surgery in order to allow immediate treatment decisions to be taken. However, the technique is contraindicated in the case of very hard tissues (bone or calcified tissue), lesions requiring more extensive study due to their complexity, or when no immediate diagnostic decision is required (3,6,7,12).
Embedding in paraffin is indicated for light microscopic evaluation, and is the most commonly used technique.
The sampled tissue is fixed in 10% formaldehyde solution. The most characteristic zones are selected from among the excess tissue resected. The specimen is processed by means of an automatized, serial hot paraffin embedding system involving progressive dehydration and final cooling and solidification, before sectioning with the microtome. The embedding process takes about 24 hours. Microtome sectioning is then carried out, mounting the specimens on slides and posteriorly removing the paraffin, with rehydration and staining as required. Finally, the specimen is again dehydrated and sealed with a coverslip. The staining techniques used depend on the type of histological study made (1).
On the other hand, embedding in methacrylate is used for electron microscopic studies, and when hard tissues or materials are involved. Electron microscopy is able to reveal a series of morphological features that distinguish normal cells from tumor cells. It is also able to establish the extent of the tumor (12).
Fresh specimen studies require transport of the biopsy sample as quickly as possible to the laboratory, for immunofluorescence evaluations. The specimens are to be humidified with saline solution to prevent drying. Before and during transport, the tissue can be kept refrigerated (2-8ºC), though freezing must be avoided (17).
Immunofluorescence techniques in turn are either direct or indirect, depending on whether the test antigen is detected with a first labeled antibody or using a second anti-antibody, respectively (18).
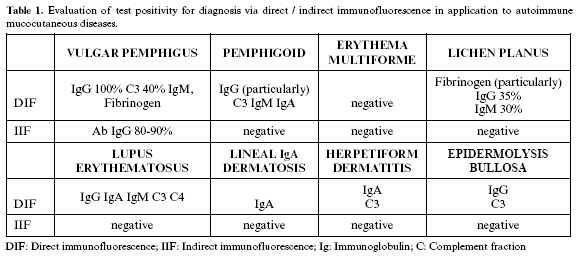
In our setting, these procedures are useful for diagnosing mucocutaneous diseases of an autoimmune nature, such as pemphigus, pemphigoid, erythema multiforme and lichen planus (Table 1).
In the case of molecular studies, genetic molecular markers allow us to detect alterations before changes in cell morphology occur or clinical manifestations develop. Analysis of such molecular alterations is objective, and aims to identify specific genetic anomalies.
The principal oral cavity carcinogens are chemical (tobacco smoke), physical (radiation) and infectious agents (papillomavirus, Candida); as mutagens, they are able to induce structural changes in genes and chromosomes through point mutations, deletions, insertion or rearrangements. However, some changes of this kind can also occur spontaneously. Such genetic alterations taking place in the context of carcinogenesis can be used as targets for the detection of tumor cells in clinical samples (6).
6) Purpose of the biopsy:
Biopsies can be performed for diagnostic or experimental purposes. Thus, apart from diagnosis with a view to treatment, a biopsy can yield important information for the histopathological study of new disease entities. With this aim in mind, studies are made in laboratory animals, and exceptionally also in humans, with the obtainment of samples that can yield valuable information on the evolution of a given disease process (3).
How to perform an oral biopsy
The materials and instrumentation required to perform an oral biopsy are not particularly sophisticated. The necessary instruments are limited to those commonly employed in surgery, such as a buccal mirror, exploratory probe, toothless dissection forceps, mosquito forceps, scalpel handpiece and number 15 blade, syringe for anesthesia, pressure forceps, scissors, periostotome, separators, needle carriers and suture mounted needles. For bone biopsies we can use gubia forceps, a chisel and mallet, a motor-driven handpiece with drills, and curettes.
As to the required material, an ejector, gauze, sterile gloves and a plastic or glass bottle containing 10% formalin solution is advised (3).
As with any surgical procedure, a biopsy requires due sterilization of the instrumentation and disinfection of the surgical field.
Most target lesions are found in soft tissues such as the tongue, cheek mucosa or lips, or in more adhered regions such as the palate and gums. Anaesthesia with a vasoconstrictor to minimize bleeding should not be applied in the actual biopsy target zone but rather at a certain distance, to avoid alterations. Thus, injection should be performed with a separation of 3-4 mm, and at the four cardinal reference points (top, bottom, left and right).
Traction suture or tissue forceps are to be used to fix the tissue to be removed. Traction sutures offer the advantage of orienting at least one lesion surface for the pathologist. Surgery is moreover facilitated, and compression or destruction of the specimen (as tends to occur when using puncture or cutting instruments) is avoided (8).
The specimen should be obtained by means of a clean and deep cut, taking care in extraction to avoid tearing or compression, as this could cause alterations. In excisional biopsies, the lesion is to be palpated carefully, determining its depth, and the incisions should slightly exceed the total depth of the lesion. In incisional biopsies, any depth within the lesion allowing the obtainment of sufficient material for study is considered acceptable. The incision should include a significant portion of the suspect tissue, though also a part of adjacent normal tissue (19).
The wound margins are subjected to debridement, with control of bleeding, and the lips of the wound are joined with suture. When a sample of gingival tissue or palate is obtained, and closure of the incision proves difficult, it can be left to heal by second intention. Oxidized and regenerated cellulose can be applied, together with gauze impregnated with tranexamic acid, to avoid bleeding (8).
After obtaining the sample, washing with physiological saline is indicated, followed by fixation. Sample processing begins once the specimen has been obtained, with the purpose of allowing tissue study under magnification. The steps comprise fixation, cutting into fragments or blocks, embedding, sectioning, staining and examination. The most common procedure is staining with hematoxylin-eosin, followed by examination under the light microscope (1).
Light microscopic studies generally involve the use of 10% formaldehyde solution in water; the concentration must be sufficient to ensure correct fixation of the tissue.
If prolonged storage of the specimens is contemplated, we can use Bouin fixating medium, which remains stable over time. The tissue sample is to be left in the fixating solution for 2-10 hours, depending on its thickness. It is then washed for 24 hours and finally immersed in 80º alcohol for posterior dehydration, clearance or embedding. The formula for preparing Bouin medium is as follows: 1.4% picric acid aqueous solution (150 ml), commercial formalin solution (50 ml) and glacial acetic acid (19 ml)(3).
Other simple fixing fluids can also be used, such as picric acid, acetic acid, chromic acid, potassium dichromate, mercury chloride, cadmium chloride, osmium tetraoxide (osmic acid), acetone or 70% alcohol - though the latter sharply dehydrates the tissues, causing artifacts, complicating epithelial staining and poorly fixing the connective tissue elements (3).
On the other hand, for electron microscopic preparations, the specimen is immersed in 3% glutaraldehyde in the refrigerator for 24 hours, followed by transfer to a 0.1 M buffer solution until study (3).
Oral cavity biopsies can give rise to complications, though in most cases such problems can be minimized by making use of a careful surgical technique. Bleeding may occur in the first 24 hours after the procedure, as a result of clot disruption during the early healing period, or secondary to suture loosening. Minor bleeding responds to local pressure application, while more important bleeding requires ligation, cauterization or the closure of some bleeding point (11).
Dehiscences are infrequent, however. Such problems may develop 5-8 days after biopsy. The implicated local factors comprise bleeding, infection, excess suture material or excessively tightened sutures that tend to strangle local vascularization (11).
On the other hand, infection is also rare and is attributable to a deficient surgical technique. Treatment in such situations consists of drainage of the infectious material, and antibiotic medication.
Another possible complication of oral biopsies is sensory impairment. This type of problem may result from a defective surgical technique, and can be avoided. Sensory defects are secondary to sensory nerve damage during the biopsy procedure. The symptoms are paresthesia of variable intensity that can persist for hours or even several months, depending on the magnitude of the damage caused (11).
The pathologist must be duly informed of the identifying data of the biopsied lesion, i.e., its macroscopic appearance, and the zone in which it is located.
The specimens obtained with oral biopsy procedures are typically small, and the risk of artifacts is considerable. These artifacts, which are sometimes seen under the microscope, may pose a problem for establishing a correct histopathological diagnosis.
Biopsy artifacts are due to defective sampling techniques, problems during transport, or incorrect processing of the tissue in the laboratory.
A small sample usually consists of a narrow strip of delicate mucosa, which tends to fold onto itself during fixation. When this happens, the junction between the epithelial and connective tissue components is usually lost - particularly if the specimen lacks an underlying submucosal or muscle tissue layer.
On one hand, when the tissue is removed with excessive force, the epithelium and connective component may suffer important damage. The forceps used to grasp the specimen may perforate the latter, leaving gaps and creating compression zones around the tissue (20-22).
On the other hand, the heat generated by an electroscalpel gives rise to alterations such as tissue protein coagulation - resulting in an amorphous epithelial and connective tissue appearance. In such situations the epithelial cells become fusiform and hyperchromatic (18,21). Some authors recommend combination of an electroscalpel and conventional scalpel. The technique in this case consists of application of the conventional scalpel for incision around the target lesion, with use of the electroscalpel to complete tissue removal. This approach affords increased hemostasia and reduces the amount of heat generated. However, the electroscalpel is generally not advised, and when it is used, a marginal incision should be made at a good distance from the tissue sampling zone, in order to avoid heat-induced changes in the target area.
Infiltrating, peripheral anesthesia is indicated, avoiding the application of excessive pressure to the tissue. Needle insertion can produce bleeding with extravasation, which tends to mask normal cell architecture. Moreover, connective tissue separation with vacuolization may occur. Therefore, infiltrating anesthesia around the target lesion is accepted if the field is sufficiently large in relation to the size of the lesion (23).
Immediate and correct fixation of the tissue specimen is necessary to interrupt autolysis and putrefaction, and to stabilize the cell proteins. A good fixing agent penetrates rapidly, preserves the cell details and hardens the sample as a protective measure. For optimum fixation, the amount of fixing agent should exceed the tissue volume by a factor of 20. Delayed fixation or a defective processing technique will cause changes in the form of cell shrinkage and cytoplasmic clustering. Furthermore, the nuclear chromatin cannot be distinguished, and the nucleoli are sometimes not visualized. Vascular, nerve and gland structures show a loss of detail, and the impression of scarring or cellularity loss is created (22).
Freezing of the tissue before fixation is not recommended (3). Freezing during transport should also be avoided, since cytoplasmic condensation has been described, secondary to cell dehydration as a result of freezing. Interstitial vacuoles form, together with vacuoles within the cell cytoplasm, due to ice crystal formation (22).
Ficarra et al. (21) recommended the following protocol to avoid possible artifacts during the surgical procedure: Firstly, good clinical judgment is required for selecting the best area for biopsy. Sufficient tissue must then be obtained with care, avoiding sample compression or traction. The sample thus obtained must be fixed immediately. The fixation bottle must be labeled with water-proof tape, and using a pencil for writing. Each specimen is to be placed in a separate bottle or container, with due identification of the different zones involved.
Posteriorly, the pathology laboratory will issue a report, identifying the material and providing details (macroscopic identification), a description of the study made, the final histopathological diagnosis, and other comments (3).
The pathologist thus may prepare the report in three different ways: certainty diagnosis, incompatibility diagnosis, or orientative diagnosis.
The certainty diagnosis is a true histopathological diagnosis. This diagnosis is stated when the findings are pathognomonic of a given type of lesion. If possible, tumor disease should specify tumor extension to the resection margins, including depth and infiltration, and the histological malignancy grade.
On the other hand, a diagnostic incompatibility report is issued when no lesions typical of a given disease entity have been observed. In these situations we must check that sampling and processing have been correct in both amount and location. In some cases there may be a previous test yielding a negative diagnosis, and the biopsy specimen in these cases serves to check the possible presence of positive lesions (24).
In contrast, the diagnosis in some cases is merely orientative and must be interpreted in close correlation to the clinical data and the findings of other complementary tests. In such cases the diagnosis is said to be compatible or suggestive of a concrete disease entity.
Finally, in some cases the pathologist is unable to draw any conclusions, and the resulting report is of a merely descriptive nature (1,3,7).
References
1. García-Peñín A. Biopsia en Cirugía Bucal. In: Donado M (eds). Cirugía Bucal: patología y técnica. Madrid: Masson; 1990.p.119-31. [ Links ]
2. Sabater-Recolons M, Viñals-Iglesias H. Las biopsias en medicina oral. Rev Europea Odontoestomatol 1997;3:175-82.. [ Links ]
3. García-Peñín A, Carrillo-Baracaldo JS, Martínez-González JM, Sada-García-Lomas JM. La biopsia en Estomatología. Rev Actual Estomatol Esp 1987;47:49-52, 55-8, 61-2. [ Links ]
4. Gandolfo S, Carbone M, Carrozzo M, Scamuzzi S. Biopsy technics in oral oncology: excisional or incisional biopsy? A critical review of the literature and the authors personal contribution. Minerva Stomatol. 1993 Mar;42(3):69-75. [ Links ]
5. Brown RS, Bottomley WK, Abramovitch K, Langlais RP. Immediate biopsy versus a therapeutic trial in the diagnosis and treatment of vesiculobullous/vesiculoerosive oral lesions. Opposing viewpoints presented. Oral Surg Oral Med Oral Pathol. 1992 Jun;73(6):694-7. [ Links ]
6. García-Núñez JA, Esparza-Gómez G, Cerero-Lapiedra R. Medios diagnósticos en medicina bucal. In: Bascones A (eds). Tratado de odontología. Tomo III. Madrid: Smithkline Beecham 1999. p. 2993-8. [ Links ]
7. Peñarrocha M, Brotons A. Biopsias. In: Peñarrocha M (eds). Cirugía Bucal. Valencia: Promolibro; 2000. p. 87-97. [ Links ]
8. Waite DE. Técnica de biopsia. In: Waite DE (eds). Tratado de cirugía bucal práctica. México: CECSA; 1984. p. 211-9. [ Links ]
9. Bramley PA, Smith CJ. Oral cancer and precancer: establishing a diagnosis. Br Dent J. 1990 Feb 10;168(3):103-7. [ Links ]
10. Shklar G. The effect of manipulation and incision on experimental carcinoma of hamster buccal pouch. Cancer Res. 1968 Nov;28(11):2180-2. [ Links ]
11. Harahap M. How to biopsy oral lesions. J Dermatol Surg Oncol. 1989 Oct;15(10):1077-80. [ Links ]
12. Pifarré S. Patología quirúrgica oral y maxillofacial. Barcelona: Jims; 1993. p. 235-98. [ Links ]
13. Lynch DP, Morris LF. The oral mucosal punch biopsy: indications and technique. J Am Dent Assoc. 1990 Jul;121(1):145-9. [ Links ]
14. López-Jornet MP. La biopsia en odontoestomatología. Descripción de la técnica mediante la utilización del punch. Revista Europea de Odonto-Estomatología 1994;3:147-50. [ Links ]
15. Eisen D. The oral mucosal punch biopsy. A report of 140 cases. Arch Dermatol. 1992 Jun;128(6):815-7. [ Links ]
16. Bermejo-Fenoll A, Lopez-Jornet P. Instrument for biopsy of oral lesions: an improved chalazion forceps. Dermatol Surg. 2006 Dec;32(12):1493-5. [ Links ]
17. Laskin DM. Cirugía bucal y maxilofacial. Buenos Aires: Panamericana; 1987. p. 529, 593-5. [ Links ]
18. Bagán JV. Enfermedades ampollares de la cavidad oral. In: Bagan JV, Ceballos A, Bermejo A, Aguirre JM, Peñarrocha M (eds). Medicina Oral. Barcelona: ed Masson; 1995. p. 220-6, 227-40. [ Links ]
19. Keszler A. Biopsies: practical standards for correct technic. Rev Asoc Odontol Argent. 1988 Jun;76(3):100. [ Links ]
20. Lolli R, Venezia A, Bellardini M, De Nisi S, Demuro G. Technical artifacts in biopsy of the oral cavity. I. Clinical and histopathologic aspects. Minerva Stomatol. 1989 Jan;38(1):37-45. [ Links ]
21. Ficarra G, McClintock B, Hansen LS. Artefacts created during oral biopsy procedures. J Craniomaxillofac Surg. 1987 Feb;15(1):34-7. [ Links ]
22. Margarone JE, Natiella JR, Vaughan CD. Artifacts in oral biopsy specimens. J Oral Maxillofac Surg. 1985 Mar;43(3):163-72. [ Links ]
23. Kearns HP, McCartan BE, Lamey PJ. Patients pain experience following oral mucosal biopsy under local anaesthesia. Br Dent J. 2001 Jan 13;190(1):33-5. [ Links ]
24. Potter TJ, Summerlin DJ, Campbell JH. Oral malignancies associated with negative transepithelial brush biopsy. J Oral Maxillofac Surg. 2003 Jun;61(6):674-7. [ Links ]
![]() Correspondence:
Correspondence:
Prof. F. Javier Silvestre-Donat
Sección de Pacientes Especiales
Clínica Odontológica Universitaria
Gascó Oliag 1
46010 - Valencia. Spain
E-mail: francisco.silvestre@uv.es
Received: 10-12-2006
Accepted: 3-05-2007