My SciELO
Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Archivos Españoles de Urología (Ed. impresa)
Print version ISSN 0004-0614
Arch. Esp. Urol. vol.60 n.4 May. 2007
Robotic renal surgery: radical and partial nephrectomy.
Amy E. Krambeck and Matthew T. Gettman.
Department of Urology. Mayo Clinic. Rochester. Minnesota. U.S.A.
SUMMARY
Objectives: By addressing the performance limitations of standard laparoscopy, robotics has developed a role in urology. While greatest use has been in pelvic prostatectomy, the role of robotics is expanding to other retroperitoneal surgeries. We review the feasibility of robotic radical and partial nephrectomy.
Methods: Medline literature search of robotic nephrectomy and partial nephrectomy was performed. Data was compiled for the review as well as personal experience.
Results: Multiple studies have demonstrated the feasibility and safety of robotic assisted laparoscopic nephrectomy and partial nephrectomy. Operative time is increased with the robotic system compared to pure laparoscopic surgery. Although complication rates are low, robotic renal surgery requires two skilled surgeons.
Conclusion: While the use of robotics in renal surgery may enhance suturing abilities, currently, this system requires two skilled surgeons and significant operative time. The exact role of robotics in extirpative renal surgery remains unclear and may require technical and surgical advancements before robotic renal surgery is widely accepted.
Key words: Robotics. Nephrectomy. Laparoscopy. Nephron-sparing surgery.
Introdution
The use of robotics in urology is well established for prostatectomy and cystectomy. The six degrees of freedom at the distal end of the instruments, three-dimensional stereoscopic vision, movement scale-down, and tremor filters enhance the performance of most experienced laparoscopists and make laparoscopy available to the inexperienced surgeon. The small, deep working space of the pelvis and the need for advanced reconstruction has made robotics suited for radical prostatectomy. The role of robotics for renal extirpative surgery; however, has been slow to develop, which is exemplified by the paucity of published robotic nephrectomy and partial nephrectomy series.
Due to the complexity and technical demand of most urologic procedures it has been difficult to incorporate minimally invasive techniques as a widespread replacement for traditional surgical approaches. Nonetheless, over recent years laparoscopic nephrectomy and even laparoscopic partial nephrectomy have emerged as the new standard of care and are often requested by the patient. Surgeons inexperienced with laparoscopy may find it time consuming and difficult to develop the skills necessary to perform a radical nephrectomy. Furthermore, the largest obstacle to the widespread use of laparoscopic partial nephrectomy is its technical difficulty. This procedure requires advanced dissection and intracorporeal suturing in a timely manner to minimize ischemic damage. The advantages of the daVinci system may theoretically limit these obstacles by decreasing the learning curve for laparoscopic procedures, making widespread availability of laparoscopic partial nephrectomy a reality.
Materials and methods
We present our preferred patient preparation, surgical technique, and postoperative care for robotic partial nephrectomy and radical nephrectomy. These techniques are devised from review of the published literature and personal experience. To date, experience with robotic radical nephrectomy and robotic partial nephrectomy has been performed using a three arm robotic system.
Robotic Radical Nephrectomy
The principals for robotic radical nephrectomy are primarily the same as those for laparoscopic radical nephrectomy. The procedure is ideal for tumors > 4 cm and confined to the kidney. Smaller, centrally located tumors not amenable to partial nephrectomy can also be considered. Patients with multiple prior abdominal surgeries may have distortion of anatomic landmarks and possible bowel adhesions; hence, consideration should be given to excluding these patients.
All patients should have adequate abdominal imaging prior to the procedure. We prefer computed tomography (CT) scan of the abdomen and pelvis. If there is a question of renal vein involvement by tumor thrombus magnetic resonance imaging (MRI) should also be performed. Standard metastatic work-up should be performed including complete blood panel, serum electrolytes, chest x-ray, liver function tests, and serum creatinine. If there is a concern of lung or bone lesions based on the above tests, then a CT scan of the chest and/or bone scan should be performed.
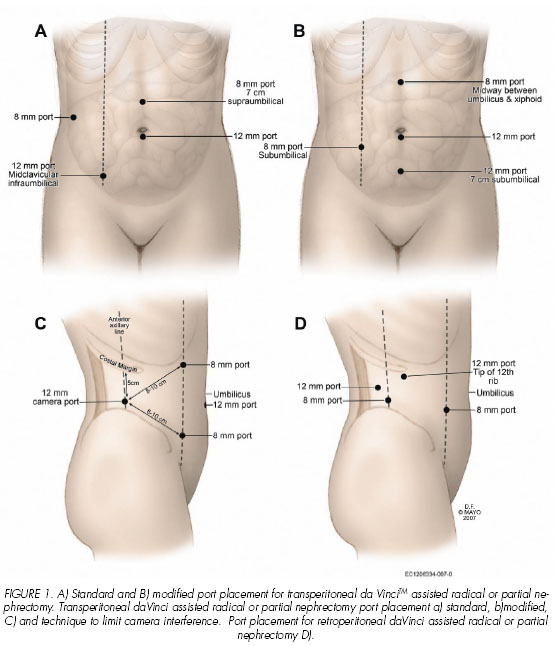
The night prior to the procedure the patient should undergo a bowel cleansing preparation. In the operating room after general anesthetic is administered a urinary bladder catheter and nasogastric tube are placed. The robotic-assisted laparoscopic nephrectomy can be performed via a transperitoneal or retroperitoneal approach. If a transperitoneal approach is to be used, the patient is placed in a 45 degree modified flank position. The ports are placed after pneumoperitoneum is established with a Veress needle technique. A 12-mm trocar is initially placed at the umbilicus to serve as the assistant port during the robotic procedure. Using the robotic endoscope through this initial port, the peritoneal cavity is inspected to determine the generalized location of the kidney and the renal lesion. A subsequent 12-mm trocar for the robotic endoscope is then placed at a midclavicular, infraumbilical position in line with the noted location of the renal tumor. Trocars for two additional robotic arms (8-mm ports, Intuitive Surgical) are then placed medially and laterally such that the distance between the camera port and each working port is at least 7 cm. To limit arm interference, the robotic ports are ideally placed with an obtuse angle between the working ports and the camera port (Figure 1A).
A modification of the port placement that has been equally effective is to place the robotic endoscope at the umbilicus and trocars for the two additional robotic arms (8-mm ports, Intuitive Surgical) are placed in the midline midway between the umbilicus and the xiphoid process at an ipsilateral midclavicular position just below the level of the umbilicus. With this trocar arrangement, the assistant port is placed in the midline approximately 5 to 7 cm below the umbilical port (Figure 1B). Recently, an alternative port placement has been described that moves the robotic camera port lateral to the working robotic ports (1). In this arrangement, a 30 degree endoscope is used in the angled up position (Figure 1C). After the working space is created, the 12 mm camera port is placed 5cm below the costal margin at the anterior axillary line. The two 8 mm working ports are placed 8-10 cm from the camera port medially toward the umbilicus such that the angle between the working ports and the camera is obtuse. An assistant 12 mm port is placed below the umbilicus in the midline. For patients undergoing a retroperitoneal approach, a full flank position is used. After the working space is created, the camera port is placed below the tip of the 12th rib, the working ports are similarly placed such that the angle between the working ports and the camera is obtuse (Figure 1D).
Once port placement is established, a hook electrode on the lateral working robotic arm and a Prograsp or Cadiere forceps on the medial working robotic arm are used for initial dissection. With a transperitoneal approach the line of Toldt is incised. The bowel is mobilized medially and for right sided tumors the duodenum is mobilized as well. If a retroperitoneal approach is used, Gerotas fascia is identified and the renal pedicle exposed. The surgical assistant facilitates dissection using conventional laparoscopic instruments to provide countertraction and suction. The renal artery and vein are identified and individually dissected bluntly. The assistant surgeon then separately divides the vessels using the 2.5 to 30 mm endovascular stapler (U.S. Surgical). The remainder of the kidney is mobilized using sharp and blunt dissection. The ureter is identified inferiorly, clipped and divided. The freed specimen is then placed in a 15-mm EndoCatch bag (U.S. surgical) by the assistant surgeon and removed intact by extending one of the midline ports approximately 7 cm.
On the first postoperative day standard serum chemistries and a complete blood count are analyzed. Ambulation is initiated on postoperative day number one and diets are advanced as tolerated with the passage of flatus. The urinary catheter is removed when the patient is fully mobile.
Robotic Partial Nephrectomy
We recommend the use of the robotic-assisted laparoscopic partial nephrectomy for predominantly solitary exophytic renal lesions, or Bosniak category III or IV cystic renal lesions. At this time we do not recommend endophytic or central renal lesions for robotic-assisted partial nephrectomy due to difficulties with dissection and preservation of the hilar structures. We also recommend exclusion of patients with multiple prior abdominal surgeries secondary to distortion of anatomic landmarks and possible bowel adhesions. Anterior and posterior lesions can be approached via a transperitoneal or retroperitoneal approach. For surgeons new to robotic-assisted procedures, we recommend initially focusing on only anterolateral-based tumors through a transperitoneal fashion as this provides more familiar anatomic landmarks and a larger working space. Once familiarity with the transperitoneal approach is achieved then retroperitoneal surgery can be attempted.
The initial work-up for partial nephrectomy candidates is the same as for radical nephrectomy. All patients should have abdominal imaging with either CT scan or MRI of the abdomen and pelvis. If there is a question of renal vein involvement by tumor thrombus MRI should be performed. Standard complete metastatic work-up should be performed including blood panel, serum electrolytes, chest x-ray, liver function tests, and serum creatinine. If there are concerns of lung or bone lesions based on the above tests then a CT scan of the chest and/or bone scan should be performed.
The night prior to the procedure the patient should undergo a bowel cleansing preparation. In the operating room after general anesthetic is administered a urinary bladder catheter and nasogastric tube are placed. The robotic-assisted laparoscopic partial nephrectomy can be performed via a transperitoneal or retroperitoneal approach with or without an intra-arterial renal catheter for renal cooling. We will describe both methods. If an intra-arterial renal catheter is to be used, it is placed prior to port placement by interventional radiology in the operating room. Port placement and patient positioning is essentially identical to that used for the radical nephrectomy. If a transperitoneal approach is to be used, the patient is placed in a 45 degree modified flank position. The ports are placed after pneumoperitoneum is established with a Veress needle. A 12-mm trocar is initially placed at the umbilicus to serve as the assistant port during the robotic procedure. Using the robotic endoscope through this initial port, the peritoneal cavity is inspected to determine the generalized location of the kidney and the renal lesion. An additional 12-mm trocar for the robotic endoscope is then placed at a midclavicular, infraumbilical position in line with the noted location of the renal tumor. Trocars for two additional robotic arms (8-mm ports, Intuitive Surgical) are then placed medially and laterally such that the distance between the camera port and each working port is at least 7 cm. In addition, the robotic ports are ideally placed with an obtuse angle between the working ports and the camera port (Figure 1A).
A modification of the port placement that has been equally effective is to place the robotic endoscope at the umbilicus and trocars for the two additional robotic arms (8-mm ports, Intuitive Surgical) are placed in the midline midway between the umbilicus and the xiphoid process at an ipsilateral midclavicular position just below the level of the umbilicus. With this trocar arrangement, the assistant port is placed in the midline approximately 5 to 7 cm below the umbilical port (Figure 1B). Likewise, the more recently reported modification of placing the robotic camera lateral to the working ports and using an angled upward endoscope has been successful for robotic partial nephrectomy. Once the working space is created, the 12 mm camera port is placed 5cm below the costal margin at the anterior axillary line. The two 8 mm working ports are placed 8-10 cm from the camera port medially toward the umbilicus such that the angle between the working ports and the camera is obtuse. An assistant 12 mm port is placed below the umbilicus in the midline (Figure 1C). 1 For patients undergoing a retroperitoneal approach, a full flank position is used. After the working space is created, the camera port is placed below the tip of the 12th rib, the working ports are similarly placed such that the angle between the working ports and the camera is obtuse (Figure 1D).
Robotic partial nephrectomy follows the same oncologic principles established for open partial nephrectomy and laparoscopic partial nephrectomy. (2-6). We choose to perform the entire procedure using the daVinci robotic system; however, some surgeons have advocated performing the initial exposure of the renal lesion with standard laparoscopic techniques and using the robotic system only for the partial nephrectomy portion of the procedure. If the daVinci system is to be used for the entire procedure, a hook electrode on the lateral working robotic arm and a Prograsp or Cadiere forceps on the medial working robotic arm are used for initial dissection. With a transperitoneal approach the line of Toldt is incised, the bowel mobilized medially and Gerotas fascia incised. If a retroperitoneal approach is used, Gerotas fascia is identified and likewise incised. The surgical assistant facilitates dissection using conventional laparoscopic instruments to provide countertraction and suction. Initially the ureter and pedicle are identified and isolated. The kidney is completely mobilized within Gerotas fascia to identify the renal lesion and exclude additional lesions. We choose visual inspection of the kidney to identify the lesion; other authors utilize a laparoscopic ultrasound probe for localization (7-8). Regardless of the technique used for localization, the fat overlying the renal lesion should be left in place and included as part of the pathologic specimen. The renal hilum is then dissected to allow occlusion during mass excision.
In preparation for the partial nephrectomy the assistant introduces a 2-O Vicryl suture (15-20 cm in length) through the 12-mm assistant port. A bolster composed of Gelfoam and Surgicel is also placed intra-abdominally in preparation for suturing after tumor excision. Mannitol 12.5 g is administered intravenously. If no intra-arterial cooling catheter is to be used, the renal artery and vein are occluded using laparoscopic bulldog clamps. This requires the surgeon at the console elevate the kidney and place the hilum on tension, while the assistant surgeon places the bulldog clamps laparoscopically. As an alternative, an additional 12 mm laparoscopic port can be placed in an infraumbilical location and a laparoscopic Satinsky clamp can be used for hilar occlusion. For patients undergoing intra-arterial catheter placement for delivery of iced saline, renal artery occlusion is achieved using the intra-arterial catheter balloon (6,9). An advantage of the intra-arterial catheter balloon is that cold ischemia can be achieved by continuous infusion of iced saline, which also prevents venous backflow during resection. Using cold, round tip scissors, the mass is then excised from the renal parenchyma. Alternatively, newer robotic hot shears can be used for tumor excision. During tumor excision, the assistant uses a suction/irrigator or conventional laparoscopic graspers for counter traction and to optimize visualization of the surgical field. The excised mass is temporarily placed adjacent to the kidney, and the assistant places two large needle drivers on the robotic arms. Collecting system defects and large bleeding vessels identified by visual inspection of the renal defect after tumor excision are controlled with 2-O Vicryl sutures. Suture closure of the renal defect is then performed using Surgicel bolsters. If a Satinsky clamp or bulldog clamps were utilized to control the renal hilum, they are then removed after closure of the renal defect. If an intra-arterial cooling balloon is used, the balloon occluding the renal artery is released after suture closure of the renal defect. The excised renal tumor is placed into a retrieval bag and frozen section evaluation is used to determine margin status at this point. Gerotas fascia is then reapproximated over the kidney with 2-O Vicryl suture, and the bowel is brought back into anatomic position. A Jackson-Pratt drain is placed through the more lateral 8-mm trocar site, and the tumor is then retrieved intact. All ports are removed under direct vision and trocar sites are closed in a standard fashion.
On the first postoperative day standard serum chemistries and a complete blood count are analyzed. Ambulation is initiated on postoperative day number one and diets are advanced as tolerated with the passage of flatus. The urinary catheter is removed when the patient is fully mobile. Prior to discharge the drain is removed pending no sign of active bleeding or urine leak.
Discussion
The advantages of the robotic system including six degrees of freedom, 3-dimensional stereoscopic vision, movement scale down and tremor filter make the system attractive to urologists with minimal laparoscopic skills. Moreover, the performance enhancing features of telerobotics may also augment the performance of the most experienced laparoscopists. The prototype of the master-slave robotic system for radical nephrectomy was first introduced by Bowersox and Cornum when they published their series of telerobotic open nephrectomy, cystotomy closures, and ureteroureterostomies (10). All procedures in this series were successfully performed with the surgeon located away from the patient and no operative complications were encountered; however, prolonged operative times were noted as a disadvantage of the robotic system (10). In 2000, Gill et al. reported the feasibility of laparoscopic telerobotic nephrectomy and adrenalectomy in the animal model using the Zeus robotic system (11). Using 5 farm pigs, laparoscopic nephrectomy and adrenalectomy was compared to telerobotic procedures. Longer operative times were noted with the robotic-assisted techniques, but the blood loss and adequacy of surgical dissection were equivalent (11). A direct comparison of the daVinci robotic system to the Zeus robotic system for laparoscopic nephrectomy, adrenalectomy and pyeloplasty was performed by Sung y cols. (12). Feasibility of the laparoscopic procedure was proven with both systems, but shorter operative times, a more favorable learning curve, and more intuitive operative movements were noted with the daVinci system. Eventually, the first laparoscopic nephrectomy in humans using the fully robotic Zeus system was reported by Guillonneau y cols. in 2001 (13). All steps of the nephrectomy were successfully performed using the robotic system with an operative time of 200 minutes and an estimated blood loss of < 100 ml. (13).
While studies have demonstrated that a decreased hospital stay and quicker convalescence is observed with robotic-assisted laparoscopic donor nephrectomies compared with open and standard laparoscopic techniques, (14). little has been published on the use of daVinci radical nephrectomy for oncologic purposes. One reason may be that the advantages of the robotic system, namely suturing and reconstruction, are not thought to be necessary for this extirpative procedure. Klinger et al. recently published their series of 5 patients with a mean tumor size of 66 cm3 range (29-120) undergoing robotic-assisted laparoscopic radical nephrectomy (15). Mean operative time was 321 minutes (range 246-437) with a mean intraoperative blood loss 150 mL (range 25-1500). Mean hospital stay was 3 days (range 1-5). One procedure was converted to a hand-assisted laparoscopic nephrectomy due to bleeding from the renal vein, but no perioperative morbidities or mortalities were noted. The authors site that the prolonged operative time may be the result of the steep learning curve of the robotic system and high body mass index (28.1) in this cohort. They further conclude that robotic nephrectomy has increased costs without a definable benefit. Nazemi et al recently published their series of 6 patients undergoing robotic assisted laparoscopic radical nephrectomy (16). In this study the authors compared robotic, laparoscopic and radical nephrectomy. They found that postoperative narcotic usage and hospital stay were higher with open surgery (p<0.05) and operative time was significantly longer with the robotic method (p=0.02). The authors also found that operating room costs were higher with the robotic and laparoscopic groups; but total hospital costs were similar among all groups (16). Based on the limited initial studies a clear benefit of robotic-assisted laparoscopic radical nephrectomy has yet to be established. However, the robotic system may be more easily mastered by the novice surgeon compared to standard laparoscopic radical nephrectomy; thus, making the procedure available to more patients in the future.
While radical nephrectomy is mainly an extirpative procedure, partial nephrectomy requires complex dissection and intracorporeal reconstruction. Nephron-sparing surgery or partial nephrectomy is increasingly being performed in the presence of a normal contralateral kidney, with proven efficacy and long-term patient related benefits (17-19). Due to difficulties with reliable, effective hemostasis, laparoscopic partial nephrectomy has been limited to highly trained surgeons at tertiary care centers. Current available hemostatic materials include argon beam coagulation, electrocautery, gelatin sponges, ultrasound dissection, radiofrequency ablation, and fibrin glue (20-25). Laparoscopic techniques mimicking open partial nephrectomy provide more reliable hemostasis but require advanced laparoscopic skills in intracorporeal suturing to limit warm ischemia time. The use of robotics has been theorized to allow the use of open partial nephrectomy hemostatic techniques with limited warm ischemia time by facilitating intracorporeal suturing.
To date only a few small series on robotic-assisted laparoscopic partial nephrectomy have been published. Our series from the Mayo Clinic, Rochester and the University of Innsbruck, Austria consisted of 13 robotic-assisted laparoscopic partial nephrectomies with 12 procedures performed purely robotically (26-27). Mean tumor size was 3.5 cm (range 2.0-6.0). An intra-arterial balloon catheter was used to cool the kidney and occlude the renal artery in 8 patients and the remaining 5 with standard renal hilar clamping. Mean operative times was 215 minutes (range 130-262), which included time for installation and setup of the daVinci system. The mean warm ischemia time was noted to be 22 minutes and cold ischemia time ranged from 18-43 minutes. Mean estimated blood loss was 17 mL (range 50-300).
We experienced no postoperative renal leaks, despite collecting system violations in two cases that were closed at time of surgical procedure. The balloon occlusion angiocatheter proved to be of great benefit. It provided excellent hemostasis and we experience no difficulties with placement, dislodgement, or renal mass excision. All procedures were completed in their entirety without open conversions. Mean hospital stay was 4.3 days (range 2-7), with one patient experiencing a prolonged hospitalization due to postoperative ileus. A positive margin on final pathologic analysis was noted in one case despite negative intraoperative frozen section analysis. Laparoscopic radical nephrectomy was subsequently performed in this patient with no evidence of residual tumor found. No recurrences have been observed in this cohort with limited follow-up of 2-11 months (26-27).
We did note that a retroperitoneal approach was possible, but it required more frequent modifications of the robotic alignment by the assistant surgeon. Therefore, we recommend a transperitoneal approach for most cases, and specifically for the novice surgeon performing their initial procedures. We also found that port placement is of the utmost importance. For optimal functioning, the angle created between each working robotic port and the camera port should be obtuse and the trajectory to the renal tumor should bisect this angle. If warm ischemia is to be used, tumor excision and repair should be less than 30 minutes to optimize renal function. By using the intra-arterial cooling catheter the ischemia time can safely be extended beyond 30 minutes; thus, facilitating a more relaxed, controlled working environment. For this reason, we feel the added time and costs of the intra-arterial cooling catheter are justified.
In a separate series Phillips et al. report their experience from the New York University School of Medicine, initially consisting of 10 patients and subsequently expanded to 12 patients (28-29). Mass exposure was performed with either the robotic system or standard laparoscopy. Mean tumor size was 1.8 cm (range not reported). The renal artery was occluded using a bulldog clamp in all cases. The renal defect was repaired first with cauterization of the base using the argon-beam coagulator or TissueLinkTM device (TissueLink Medical, Inc., Dover, NH), then placement of Fibrinogen-soaked Gelfoam (Pfizer, New York, New York) with activated thrombin, and finally suture closure. Mean operative time was 265 minutes (range not reported). Cold ischemia was not performed in this series and average warm ischemia time was 26 minutes (range not reported). Mean blood loss was 240 mL (range not reported).
Collecting-system repair was necessary in 25% of the cases with one patient developing a postoperative urine leak that was managed with a percutaneous drain for 5 weeks. Conversion to open technique was necessary for two patients in this series due to robot malfunction in one and vascular clamps dislodged during mass excision in the other. A third patient required conversion to hand-assisted laparoscopy due to bleeding after removal of the hilar clamps. Mean length of stay was 2.7 days (range not reported). Margin status was not reported in this series (28-29).
Both series found the robotic-assisted laparoscopic partial nephrectomy to be feasible and that suture closer of the renal defect was possible using the daVinci system. It was also recognized by both series that unlike standard laparoscopy, a highly skilled assistant surgeon familiar with laparoscopy is imperative. It is the assistant surgeons responsibility for application of the pedicle clamps to the hilum, to exchange the instruments and aid in hemostasis. If an emergent conversion is necessary it must be initiated by the assistant surgeon while the primary surgeon scrubs. It can also be challenging for the secondary surgeon to provide assistance due to the location and movement of the robotic system. Furthermore, we have found that an operating room staff familiar with robotic equipment and set-up is essential for the procedure to be performed smoothly and in a timely manner.
The NYU group further compared the robotic-assisted laparoscopic partial nephrectomy to standard laparoscopic partial nephrectomy (8). They evaluated 10 patients with a mean tumor size of 2.0 cm treated with robotic assisted laparoscopic partial nephrectomy and 10 patients with a mean tumor size of 2.18 cm treated with laparoscopic partial nephrectomy. In their comparison no statistically significant differences in operative time, ischemic time, estimated blood loss, hospital stay, change in serum creatinine, or change in hematocrit was noted between the two groups. There were 2 intraoperative complications in the robotic group requiring conversion to hand assisted laparoscopy in one and open procedure in the other. There were no positive margins in the robotic group and one positive margin in the standard laparoscopic group. The authors conclude that no real advantage is noted with the robotic system over standard laparoscopy. They also warn that because the primary surgeon is seated away from the table the threshold for procedure conversion may be lower when a complication is encountered during a robotic procedure (8).
One of the biggest technical challenges for robotic renal surgery has been optimal port placement. Kaul y cols. recently reported their initial experience with robotic partial nephrectomy in 10 patients with a mean tumors size of 2 cm. (1). Similar to other institutions experience with robotic partial nephrectomy, the authors found the technique was feasible. The mean console time was 158 minutes, the mean warm ischemia time was 21 minutes, and the mean hospital stay was 1.5 days. One patient required a blood transfusion postoperatively and one urine leak was noted. In comparison to other reports, the authors stress placement of the camera port lateral to the robotic working ports and use a 30 degree upward optic to facilitate visualization (Figure 1D). With this arrangement, additional visualization and mobility within the visual field is permitted and the plane of motion of the robotic arms is different from that of the camera arm.
While feasibility of robotic renal surgery has been proven, additional disadvantages of the daVinci system that ultimately must be addressed include cost, the steep learning curve and the set-up time. The lack of tactile feedback (haptics) makes the surgeon reliant on visual cues and may hamper the inexperienced laparoscopist. However, with time and technological advancements we feel theses limitations with be remedied as has been observed with radical prostatectomy. At this time, further experience and ongoing technical modifications are necessary to streamline use of robotics for minimally invasive oncologic renal surgery.
Conclusion
Although studies have demonstrated that robotic-assisted laparoscopic radical nephrectomy and partial nephrectomy are feasible and associated with minimal complications, definitive roles in the general urologists practice remain to be defined. Robotic-assisted radical nephrectomy at this point has not demonstrated a clear advantage over laparoscopic radical nephrectomy. These findings most likely result from the extirpative nature of the procedure. Robotic-assisted laparoscopic partial nephrectomy has also been successfully performed. Although warm ischemia time is reduced by enhanced intracorporeal suturing using the daVinci system, a skilled surgical assistant is required at the present time for the success of the procedure. As additional technical modifications are introduced, this requirement may not be as important thereby potentially increasing the appeal for practicing urologists. Nonetheless, for robotic-assisted radical and partial nephrectomy to become a routine part of the urologists general practice, further modifications in surgical technique and equipment may be necessary.
![]() Correspondence:
Correspondence:
Amy E. Krambeck, M.D.
Department of Urology
Mayo Clinic
200 First Street Southwest
Rochester, Minnesota 55905 (USA)
krambeck.amy@mayo.edu
References and recommended readings (*of special interest, **of outstanding interest)
*1. KAUL, S. ; LAUNGANI, R. ; SARLE, R. y cols.: da Vinci-assisted robotic partial nephrectomy: technique and results at a mean of 15 months of follow-up. Eur Urol.; 51: 186-191; 2007. [ Links ]
2. GILL, IS.; DESAI, MM.; KAOUK, JH. y cols.: Laparoscopic partial nephrectomy for renal tumor: duplicating open surgical techniques. J. Urol. 167: 469-476; 2002. [ Links ]
3. DESAI, MM.; GILL, IS.; KAOUL, JH. Y cols.: Laparoscopic partial nephrectomy with suture repair of the pelvicaliceal system. Urology. 61: 99-104 ; 2003. [ Links ]
4. RASSWEILER, JJ. ; FREDE, T. ; RECKER, F. y cols. : Retroperitoneal laparoscopic nephropexy. Urol Clin North Am. 28: 137-44; 2001. [ Links ]
5. BERMUDEZ, H. ; GUILLONNEAU, B. ; GUPTA, R. y cols. : Initial experience in laparoscopic partial nephrectomy for renal tumor with clamping of renal vessels. J Endourol. 17: 373-378; 2003. [ Links ]
6. JANETSCHEK, G.; ABDELMAKSOUD, A.; BAGHERI, F. y cols.: Laparoscopic partial nephrectomy in cold ischemia: renal artery perfusion. J Urol. 171: 68-71; 2004. [ Links ]
7. PHILLIPS, CK.; TANEJA, SS.; AND STIFELMAN, MD. Robot-assisted partial nephrectomy: the NYU technique. J Endourol. 19: 441-445; 2005. [ Links ]
*8. CARUSO, RP.; PHILLIPS CK.; KAU, E. y cols.: Robot assisted laparoscopic partial nephrectomy: initial experience. J Urol. 176: 36-39; 2006. [ Links ]
9. MARBERGER, M.; GEORGI, M.; GUENTHER, R. y cols.: Simultaneous balloon occlusion of the renal artery and hypothermic perfusion in in situ surgery of the kidney. J Urol. 119: 463-467; 1978. [ Links ]
10. BOWERSOX, JC.; CORNUM, RL. Remote operative urology using a surgical telemanipulator system: preliminary observations. Urology, 52: 17-22; 1998. [ Links ]
*11. GILL, IS.; SUNG, GT.; HSU, TH. Y cols.: Robotic remote laparoscopic nephrectomy and adrenalectomy: initial experience. J Urol. 164: 2082-2085; 2000. [ Links ]
12. SUNG, GT.; GILL, IS. Robotic laparoscopic surgery: a comparison of the daVinci and Zeus systems. J Urol. 58: 893-898 ; 2001. [ Links ]
*13. GUILLONNEAU, B. ; JAYET, C. ; TEWARI, A. Y cols.: Robotic assisted laparoscopic nephrectomy. J Urol. 166: 200-201; 2001. [ Links ]
14. HORGAN, S.; VANUNO, D. Robotis in laparoscopic surgery. J Laparoendosc Adv Surg Tech A. 11: 415-419; 2001. [ Links ]
**15. KLINGLER, DW.; HEMSTREET, GP.; BALAJI, KC. Feasibility of robotic radical nephrectomy; initial results of single-institution pilot study. Urology. 65: 1086-1089 ; 2005. [ Links ]
**16. NAZEMI, T. ; GALICHI, A. ; STERRETT, S. Y cols. : Radical nephrectomy performed by open, laparoscopy with or without hand-assistance or robotic methods by the same surgeon produces comparable perioperative results. International Braz J Urol. 32: 15-22; 2006. [ Links ]
17. LAU, W.; BLUTE, M.L.; WEAVER, A.L. y cols.: Matched comparison of radical nephrectomy vs. nephron-sparing surgery in patients with unilateral renal cell carcinoma and a normal contralateral kidney. Mayo Clin Proc. 75: 1236-1242; 2000. [ Links ]
18. HERR, HW. Partial nephrectomy for unilateral renal carcinoma and a normal contralateral kidney: 10-year followup. J Urol. 161: 33-34; 1999. [ Links ]
19. FERGANY, AF.; HAFEZ, KS.; NOVICK, AC. Long-term results of nephron-sparing surgeryf or localized renal cell carcinoma: 10-year followup. J Urol. 163: 442-445; 2000. [ Links ]
20. ORGAN, K.; CADEDDU, JA. Minimally invasive management of the small renal tumor: review of laparoscopic partial nephrectomy and ablative techniques. J Endourol. 16: 635-643 ; 2002. [ Links ]
21. GUILLONNEAU, B.; BERMUDEZ, H.; GHOLAMI, S.; y cols. : Laparoscopic partial nephrectomy for renal tumor: single center experiency comparing clamping and no clamping techniques of the renal vasculature. J Urol. 169: 483-486; 2003. [ Links ]
22. SIMON, SD.; FERRIGNI, RG.; NOVICKI, DE. Y cols.: Mayo Clinic Scottsdale experience with laparoscopic nephron sparing surgeryf or renal tumors. J Urol. 169: 2059-2062; 2003. [ Links ]
23. JANETSCHEK, G.; DAFFNER, P.; PESCHEL, R. y cols.: Laparoscopic nephron sparing surgery for small renal cell carcinoma. J Urol. 159: 1152-1155; 1998. [ Links ]
24. HARMON, WJ.; KAVOUSSI, LR.; BISHOFF, JT. Laparoscopic nephron-sparing surgery for solid renal masses. Urology. 56: 754-759; 2000. [ Links ]
25. RICHTER, F.; SCHNORR, D.; DEGER, S. y cols.: Improvement of hemostasis in open and laparoscopically performed partial nephrectomy using a gelatin matrix-thrombin tissue sealant (FloSeal). Urology. 61: 73-77; 2003. [ Links ]
**26. GETTMAN, MT.; BLUTE, ML.; CHOW, GK. Y cols.: Robotic-assisted laparoscopic partial nephrectomy: technique and initial clinical experience with daVinci robotic system. Urology. 64: 914-918; 2004. [ Links ]
*27. PESCHEL, R.; NEURURER, R.; BLUTE, ML. y cols.: Robotic-assisted laparoscopic partial nephrectomy. J Urol. 171 (4 Suppl): 471; 2004. [ Links ]
*28. TANEJA, SS.; CARUSO, RP.; PHILLIPS, CK. Y cols.: Robotic partial nephrectomy: initial experience. J Urol. 171(4 Suppl): 339; 2004. [ Links ]
**29. PHILLIPS, CK.; TANEJA, SS.; STIFELMAN, MD. Robotic-assisted laparoscopic partial nephrectomy: the NYU technique. J Endourol. 19: 441-445; 2005. [ Links ]











 text in
text in