INTRODUCTION
Cancer is the second most common cause of death after cardiovascular disease worldwide (1). Pancreatic cancer has a very poor prognosis and is the fourth most common cause of death among other cancer types in the USA (2). The disease is rarely seen before the age of 45 years but the incidence increases with age. The disease is most frequently seen at the ages between 65 and 69 years for men, and between 75 and 79 years for women according to the Global Disease Burden Study (3).
Despite recent advances in medicine over the last century, the survival of patients with pancreatic cancer improved only a little (4). Pancreatic cancer is one of the rare cancer types with a mortality rate of 100 %. It shows the shortest survival time when we consider all cancer types (5,6). It has the poorest prognosis among all solid tumors (7). Patients with pancreatic adenocarcinoma have an average 5-year survival of 10 % to 15 % (8).
Factors such as age, tumor size, disease stage, lymph-node metastasis, tumor grade, and serum CA19-9 levels have been shown to be associated with survival in patients with pancreatic adenocarcinoma (9,10). New prognostic markers for patients with pancreatic cancer are still under investigation. Recently, some studies suggested that malnutrition, sarcopenia, and inflammation might have an effect on survival in patients with malignancies (11-17). Malnutrition, defects in inflammatory response, and reduction in muscle mass can cause increased sensitivity to infection, delay in wound healing, and postoperative complications (11-13). Sarcopenia is defined as a reduction in muscle mass and muscle strength. In some studies it was seen that cancer patients with sarcopenia had worse prognoses (14-17). We aimed to investigate the possible association between psoas muscle mass, inflammation, nutritional status at the time of diagnosis, and survival in patients with pancreatic adenocarcinoma.
MATERIAL AND METHODS
This retrospective study included 219 consecutive patients with pancreatic adenocarcinoma who were admitted to the medical oncology outpatient clinic of a university hospital between January 2015 and January 2019. Inclusion criteria were a diagnosis of pancreatic cancer based on a pathology specimen and/or radiological imaging, and age older than 18 years. Patients were excluded from the study if they were pregnant, diagnosed with another malignancy, or had infection, chronic inflammatory disease, liver cirrhosis, proteinuria, steroid usage, muscle disease, or any other disease that can cause sarcopenia, malnutrition and hypoalbuminemia.
Clinical, laboratory, and demographic data were obtained from patient records at the hospital. Psoas muscle mass, nutritional status, and inflammation status were determined and collected from laboratory results and measurements at the time of the first visit. All the data collected were those recorded when they were first diagnosed with pancreatic adenocarcinoma, in the pre-treatment period. The association between psoas muscle mass, sarcopenia, inflammation status, nutritional status at the time of diagnosis, and prognosis was investigated. The study was approved by the local university ethics committee.
ASSESSMENT OF NUTRITIONAL STATUS
The Prognostic Nutritional Index (PNI) was used to assess the nutritional status of patients. The PNI was calculated from serum albumin and lymphocyte count values. The calculation was performed by using the suggested formula - 10 x serum albumin (g/L) + (5 x lymphocyte count (109/L)). According to reference data, a PNI lower than 45 was considered malnourishment (18).
ASSESSMENT OF INFLAMMATION
Leucocyte count, and neutrophil-to-lymphocyte ratio (NLR) from a complete blood count, which were routinely performed during outpatient clinic follow-up visits, were used.
PSOAS MUSCLE MASS MEASUREMENT
Right psoas muscle mass was calculated from sections at the level of the 3rd lumbar vertebra using the abdominal computed tomography (CT) scans carried out for diagnosis and staging. Measurements were made by an experienced radiology specialist. The collected data were evaluated for each gender separately, and patients below the 25th percentile were deemed sarcopenic (19). CT scans were performed using a Somatom Definition AS 128-Slice (Siemens, Germany). Parameter adjustments included 5 mm as sequence thickness, 2 as throb, 5 mm as restructuring interval, and 320-400 mm as FOV (depending on patient size). Staging was performed by using the 2010 World Health Organisation TNM classification for pancreatic exocrine tumors (20).
STATISTICAL ANALYSIS
The NCSS (Number Cruncher Statistical System) 2007 (Kaysville, Utah, USA) program was used for the statistical analysis. While evaluating the study data, in addition to descriptive statistical methods (mean, standard deviation, median, 25th-75th percentile, frequency, ratio, minimum and maximum) the distribution of the data was evaluated using the Shapiro-Wilk test. The Mann-Whitney U-test was used to compare two groups not showing a normal distribution of quantitative data. A regression analysis was used to determine the effect of independent variables on the dependent variable. The Kaplan-Meier method was used to compare survival processes as a group. Significance was evaluated as p < 0.05.
RESULTS
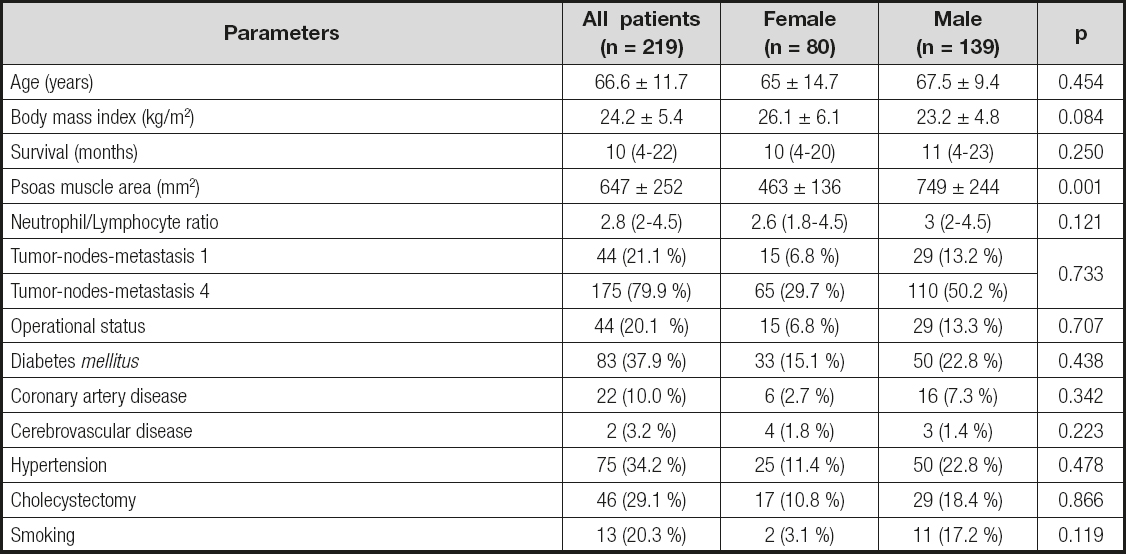
The mean age of patients (80 female and 139 male) was 66.6 ± 11.7 years. The male/female ratio was 1.73. Median psoas muscle area, leucocyte count, and NLR were 599 mm (447-801), 7.70 K/µL (5.98-9.37), and 2.84 (2.02-4.53), respectively. Mean PNI of patients was 45.05 ± 7.8. The patients were at stage 1 or stage 4 according to the tumor-nodes-metastasis (TNM) classification. The demographic and laboratory characteristics of patients are shown in table I. Mean psoas muscle area stratified by gender was statistically lower for female than male subjects (463 ± 136 vs. 749 ± 244 mm2, p < 0.001).
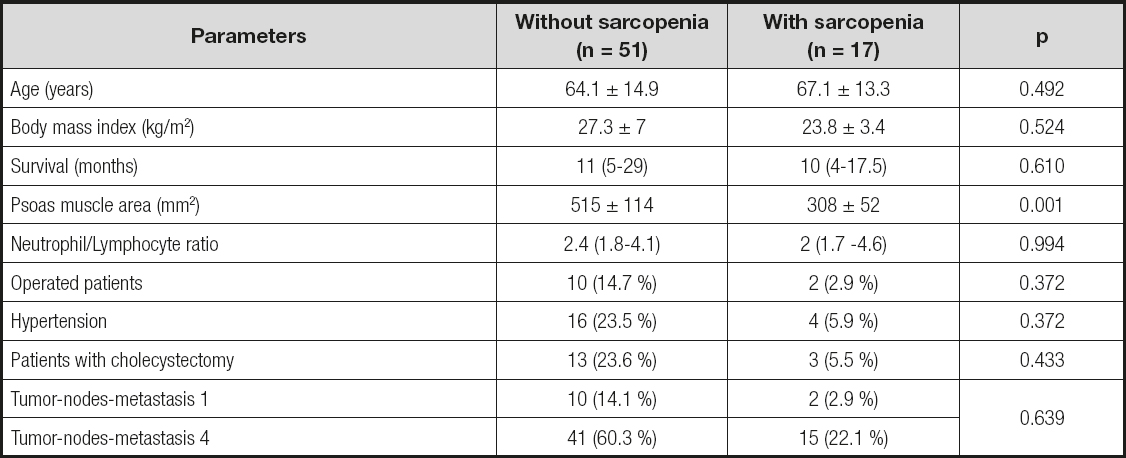
Psoas muscle mass was measured using abdominal CT scans in 191 patients (87.2 %) out of the 219 patients included in the study. Sarcopenia was found in 25.1 % (48) of patients in whom psoas muscle mass was measured. There was no significant relationship between sarcopenic status and age, body mass index, survival, NLR, operational status, hypertension, cholecystectomy, and TNM classification (p > 0.05) (Table II and Table III). The characteristics of the patients with and without sarcopenia are shown in table II and table III.
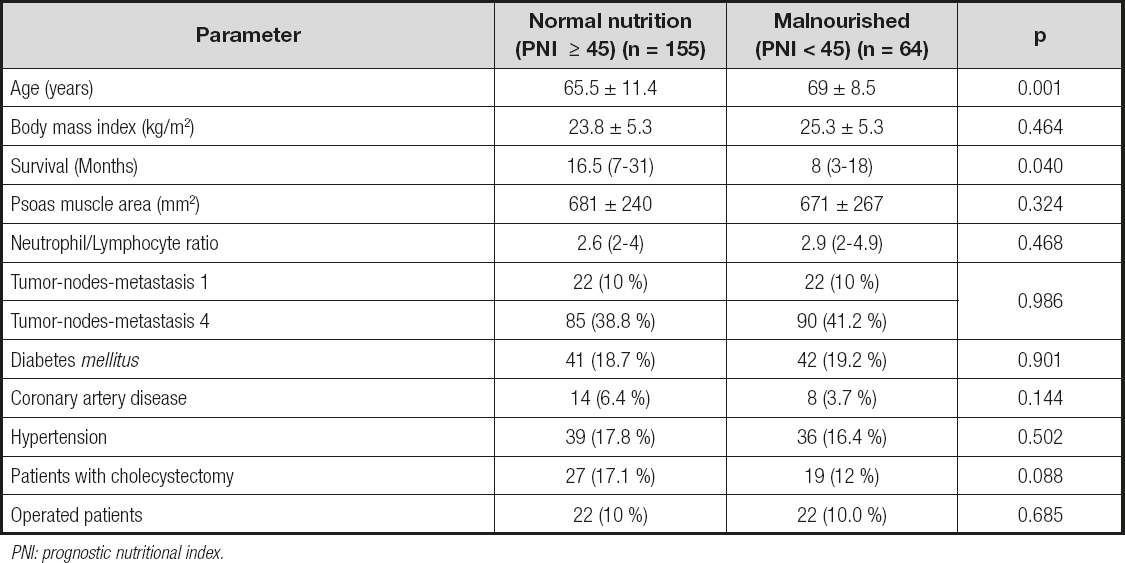
Malnutrition was found in 30 % (n = 64) of the patients according to PNI, while 70 % (n = 155) were found to have a normal nutritional status (Table IV). The mean age of patients with normal nutrition was significantly lower (65.5 ± 11.4) than that of malnourished patients (69 ± 8.5) (p = 0.001) (Table IV). Malnourished patients had a significantly shorter survival (median 8 (3-18) months) when compared to patients with normal nutrition (median 16.5 (7-31) months) (p = 0.04) (Table IV, Fig. 1). Table IV shows the comparison of parameters according to patient nutritional status.
Table IV. Comparison of parameters according to the nutritional status of patients
PNI: prognostic nutritional index.
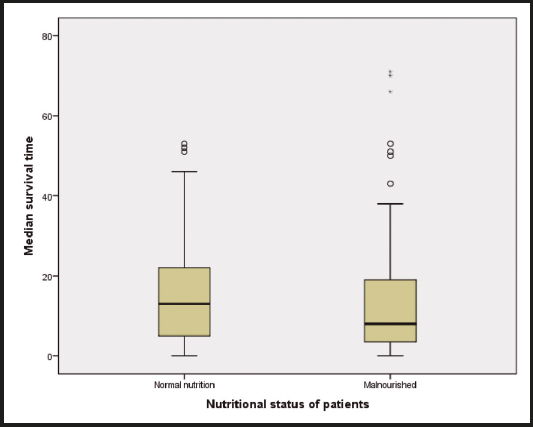
Figure 1. Median survival time as stratified by patient nutritional status was statistically lower for malnourished than for well nourished subjects (8 (3-18) vs. 16.5 (7-31), p = 0.040).
A Kaplan-Meier survival analysis of patients with pancreatic adenocarcinoma and the effect on survival stratified by nutritional status showed that patients with malnutrition have significantly shorter survival periods (log-rank, p < 0.001) (Fig. 2). A multiple linear regression analysis that was performed to evaluate the effects of parameters on survival was found to yield statistically significant results (F (9.41) = 2.406, p < 0.001). Independent variables in the model explain 34.6 % of total variance in survival (R2 = 0.346, p < 0.001). When regression coefficients were analyzed, it was seen that factors such as PNI score ≥ 45 (β = -0.146, p < 0.05), having stage-1 disease (β = 0.331, p < 0.05), having low CA19-9 levels (β = -0.152, p < 0.05), and younger age (β = -0.171, p < 0.05) have a significantly positive effect on survival (Table V).
DISCUSSION
The present study revealed that the presence of malnutrition, high levels of CA19-9, advanced disease, and advanced age at the time of diagnosis significantly affect survival negatively in patients with pancreatic carcinoma. On the other hand, there was no significant relationship between patient inflammation status, psoas muscle mass, sarcopenia, and survival.
Malnutrition is frequently seen in patients with malignancy. The severity of malnutrition varies according to cancer type, location, and stage in these patients. Loss of weight and appetite were found in nearly half of newly diagnosed patients, and in 75 % of patients with advanced-stage disease (21). Malnutrition is highly prevalent in patients with pancreatic cancer (22). Conditions such as anorexia, nausea, vomiting, abdominal distension, pain, and infection caused by the disease may lead to malnutrition (23). The extent of weight loss is vitally important for prognosis. The presence of weight loss before treatment onset is associated with shorter survival (22). We used the Onodera Prognostic Index to evaluate the nutritional status of patients (18). The current study showed that 30 % of patients had malnutrition. The mean age of patients with malnutrition was significantly higher. And malnourished patients had a significantly shorter survival time. Our findings are compatible with those of similar studies in the literature, revealing a longer survival in patients with no malnutrition (22,24,25).
Recently, researchers focused on sarcopenia and its association with survival in patients with malignancy. Various methods such as body imaging techniques, bioimpedance analysis, and anthropometric measures are used to analyze sarcopenia, and studies are still ongoing to find out the gold standard method. We analyzed the association between sarcopenia and survival, and could not find any significant relationship between these neither in male nor in female patients. In contrast, some studies suggest a shorter survival in patients with sarcopenia (26). This difference can be caused by the use of different methods for the diagnosis of sarcopenia. In various studies, while sarcopenia was evaluated, it was standardized for height by dividing by height squared. Since data related to patient height were missing in the records used for our retrospective study, the data were calculated separately for both genders, and values < 25th percentile were considered sarcopenic.
There is no gold-standard screening test for pancreatic cancer yet. However, CA 19-9 is a well-accepted laboratory test for screening pancreatic cancer with a sensitivity of 80 %, and a specificity of 90 % (27). On the other hand, CA19-9 levels are prone to increase not only in pancreatic adenocarcinoma but also in hepatocellular cancer, biliary duct malignancies, and rarely in cases of gallstones, cholangitis, pancreatitis, cirrhosis, and other malignancies (i.e., gastric, ovary, colorectal, breast, uterine cancers) (27). The high sensitivity and specificity rates of CA 19-9 for pancreatic cancer are closely related to the diameter of the tumor. Unfortunately, their efficiency is limited, especially for detecting small, surgically resectable tumors (28). Serum CA 19-9 level is associated with resectability and long-term prognosis (28). When we analyzed the regression coefficients in our study, it was found that low levels of CA 19-9 had a significantly positive effect upon survival.
High neutrophil counts cause progression of neoplasia by releasing various growth factors and maintaining a suitable environment for tumor formation (29). In some studies, it has been suggested that the presence of inflammation may affect prognosis in patients with pancreatic cancer (30). Iwai N. et al. reported that high NLRs may be an independent indicator of poor prognosis in patients with unresectable pancreatic cancer (30). However, we could not find a significant relationship between NLR at the time of diagnosis and survival in our study.
It is difficult to treat pancreatic cancer, which has a high mortality rate (4,5). In all, 90 % of patients are inoperable, and 40-50 % of these are found in a locally advanced stage. Mean survival time is 6-10 months, but unfortunately this time is only 3-6 months for metastatic cases (31). Thus, diagnosing pancreatic cancer in early stages is of vital importance for survival and operability. In the present study, mortality rates were found to be 21.1 % for patients with stage-1 disease, and 78.9 % for patients with stage-4 disease. These findings are in line with those of similar studies in the literature, revealing a longer survival for patients with early-stage disease (4,5). Also in our study, the analysis of regression coefficients showed that a diagnosis of stage-1 disease had a significantly positive effect upon survival.
In conclusion, the present study showed that younger age, early-stage disease, low CA19-9 levels, and PNI score ≥ 45 were associated with longer survival in patients with pancreatic cancer. No significant relationship was found between survival rates and psoas muscle mass, presence of sarcopenia, and NLR at the time of diagnosis. We consider that a prospective study including various parameters related to sarcopenia, inflammation, and malnutrition would be beneficial for a better understanding of this issue.