INTRODUCTION
The obesity prevalence in Mexican women has increased dramatically by 30.6 %, from 2000 to 2018; presently, more than 75 % of women of reproductive age have a body mass index (BMI) above 25 kg/m2 (1). Around 30 % of women in the United States of America are expected to have a BMI ≥ 30 kg/m2 when they become pregnant (2), while in Mexico this number is unknown at the national level, but as a proxy, 36 % of women between 20 to 50 years old presents obesity (> 30 kg/ m2) (1).
The evidence had supported the suitability of achieving a healthy weight gain for positive pregnancy outcomes (3). Pregnant women living with obesity face elevated risks of adverse outcomes during pregnancy and childbirth, including gestational diabetes, hypertension, pre-eclampsia, and giving birth to a macrosomic baby, which leads to a higher risk of complications, such as obstructed labor and postpartum hemorrhage (4,5). Furthermore, systematic review and meta-analyse has shown that maternal overweight and obesity are associated with pre-term and post-term delivery (6,7), which also lead to future complications for offspring.
Recently, it has been recognized that overweight may contribute to low iron levels, as BMI is negatively correlated with serum iron (8), and iron deficiency is more common in overweight than normal-weight women (9,10). This correlation has been documented in Mexico since 1999 when the National Nutrition Survey showed that the risk of iron deficiency in women of reproductive age living with obesity was 2-4 times higher compared to individuals with a healthy weight at similar dietary iron intakes (11).
Some studies in pregnant women have shown that a high BMI, pre-gestational as well as during pregnancy, influences iron stores negatively (12,13), and that pre-pregnant obese women have lower ferritin concentrations than pre-pregnant non-obese women (12,14-16).
Concurrently, a significant proportion of Mexican women of reproductive age are anemic (34.3 %), and this proportion has been increasing lately, particularly in women from households at lower socioeconomic level (17). Anemia in pregnancy warrants concern as it impacts negatively on the development of the fetus and increases the risk of low birth weight leading to an elevated risk of adverse, metabolic long-term health outcomes in the offspring (18).
Thus, while obesity and anemia may seem disentangled and their presence almost “antagonistic”, approximately 10 % of women of reproductive age in Mexico are both obese and anemic (19). Also, obesity and anemia often share environmental conditions and behaviors, and can co-occur at the household and individual level; this co-existence is an example of what has been named the “double burden of malnutrition” (20).
In Mexico, high proportion (98.7 %) of women attend antenatal care visits (21), also Mexican medical guidelines for antenatal care consider monitoring healthy weight gain during pregnancy (monitoring weight at each visit), early identification of anemia (through the Hb (hemoglobin) determination levels), and iron supplements prescription in pregnant women at anemia risk. Despite that, as both conditions, obesity, and anemia are highly prevalent in Mexico, there is a necessity to study their impact on mother-child health together.
This study has two aims: first, to identify if pre-pregnancy BMI and gestational weight gain (GWG) are associated with Hb levels at the third trimester; additionally, identify if the combination of BMI status and anemia at delivery will influence neonatal outcomes.
MATERIALS AND METHODS
DESIGN AND SETTING
The study is based on an ongoing birth cohort study (referred in Spanish, MAS-Lactancia) which was initiated in 2016, and has so far recruited 980 pregnant women. Written informed consent for inclusion in the cohort was obtained from all the participating pregnant women. The study is being carried out by the National Institute of Public Health (INSP) in collaboration with the Mexican Institute for Social Security (IMSS) in Cuernavaca, Morelos. The INSP Ethics in Research Committee and its Research commission (# approval 1281), as well as the National Committee of Scientific Research of IMSS (#R2015-785-107) approved the protocol of the cohort study.
PARTICIPANTS
Pregnant women were considered for inclusion in the study if they were between 18 to 39 years old, with a gestational age between 16 to 22 weeks. All participating women were affiliated to the IMSS, one of the Institutions in the Mexican Health System that provides health services for employees (mainly for the private sector) and their families, who typically belong to the middle socioeconomic strata. Exclusion criteria for the cohort were women with (1) more than one fetus, (2) high-risk pregnancy such as preeclampsia or gestational diabetes, (3) renal, liver, heart, or cardiovascular disease, and (4) endocrine disorders More details of the cohort are described elsewhere (22).
For the first aim of this analysis, we included only 108 pregnant women, for whom information on Hb levels and repeated weight measures recorded in the IMSS-medical files (Medical System of Family Medicine) were available. For the second aim, we included 63 offspring in an unbalanced panel, as they had a follow-up until 3 months old. Figure 1 presents the analytical sample of this analysis derived from the original cohort.
MAIN INDEPENDENT VARIABLES
We reviewed each participant´s clinical files from the first antenatal visit to delivery and recorded pre-pregnancy weight (self-reported), and weight reported from each antenatal visit with the corresponding gestational age (determined from the first day of the last menstrual period). Anthropometric measurements (weight and height) were taken using Lohman's Anthropometry Manual as Reference (23) by the cohort's trained personnel and standardized at the beginning of the study. Maternal weight was measured on an electronic scale (Tanita, model 1582, Illinois, USA) with an accuracy to the nearest 10 g. Standing height was measured using a Shorr stadiometer (SmoothSlide©, USA) with an accuracy to the nearest 1mm. All measurements were done twice, and the average was used in the models. Pre-pregnancy and delivery BMI were calculated and categorized into normal pre-pregnancy BMI (≥ 18.5-24.9 kg/m2, N-BMI), overweight (≥ 25-29.9 kg/m2, OV-BMI), and obesity (≥ 30 kg/m2, OB-BMI) (24). GWG was categorized according to the Institute of Medicine (IOM) guidelines based on pre-pregnancy BMI category as insufficient (N-BMI < 11.5 kg, OV-BMI < 7 kg, OB-BMI < 5 kg), excessive (N-BMI > 16 kg, OV-BMI > 11.5 kg, OB-BMI > 9 kg), and adequate in the range between the two mentioned categories (25).
OUTCOME VARIABLES, PREGNANT WOMEN
We obtained the Hb levels information from medical files. Hb levels were analyzed at the IMSS laboratory facilities (by UniCel DxC 600/800 SYNCHRON) and recorded in the files with the corresponding gestational age. We made an altitude adjustment of Hb levels by the municipality of residence (Table I) in order to classify pregnant women with anemia according to the World Health Organization (WHO) Hb classification (Hb < 11 g/dL) (26).
OUTCOME VARIABLES, OFFSPRING
Gestational age at birth was estimated based on the first day of the last menstrual period, and classified according to the American College of Obstetrics and Gynecologists as (27).
For anthropometric measurements, the child´s weight and length were measured at birth (in the delivery room), at 1 and 3 months of age using standardized procedures (23). Measures at birth were corroborated (during the first three days of life) with the measures done by the cohort´s trained personnel. A portable electronic pediatric scale (Tanita BABY MOMMY model 1582, Illinois, USA) with precision to the nearest 10 g, was used to measure weight. A wooden infantometer (SmoothSlide©, USA) with a precision of 1mm was used to measure height. All measurements were taken twice, and we used the average for estimations. WHO-Anthro software (v.3.2.2, 2011) was used to estimate Z-scores of weight-for-age (WAZ), length-for-age (LAZ), and weight-for-length Z-score (WLZ) based on WHO growth standards (28). Triceps skinfold was measured at 1 and 3 months by trained staff using a Lange skinfold caliper with precision of 0.5 mm.
COVARIATES
We also obtained information from clinical files and from questionnaires administered to pregnant women by trained personal (at recruitment) on the following variables: age, education (total years studied), socioeconomic status (tertiles), being of indigenous origin (by self-identification of participant or speaking an indigenous language), parity (1st pregnancy or other), marital status (married/with a partner or single/divorced), type of employment (housewife/none, student, informal worker, formal worker), and type of delivery (C-section or vaginal). The information on supplements/multivitamins type consumed during pregnancy was obtained during second and third trimesters.
STATISTICAL ANALYSIS
Descriptive statistics are presented for variables at baseline, delivery, and post-partum as means (SD), proportions, and stratified by pre-pregnancy BMI classification. Bi-variate hypothesis tests were performed by means with ANOVA test for continuous variables and Fisher´s exact test for categorical variables.
An adjusted linear regression model for longitudinal data (considering unbalance panel) was fitted to estimate Hb levels according to GWG (insufficient, adequate, and excessive) and stratified by pre-pregnancy BMI (normal, overweight, or obese). The model was adjusted for age, education, parity, supplement intake, and gestational age (trimester).
Then, we identified at the end of pregnancy six combinations between BMI classification (normal, overweight or obesity), and anemia (yes/no). We obtained the proportion of women in the mentioned classification and related them to the proportion of delivery method and the gestational age classification at birth. Also, the six combinations were used to compare in terms of the mean anthropometric measures at birth, 1 and 3 months post-partum using regression models adjusted for sex. Finally, adjusted models were run to estimate the effect of the combination of BMI status and anemia at the end of pregnancy with the offspring´s repeated anthropometric measures (longitudinal data for the anthropometric measures) were fitted to estimate differences in growth trajectories between the six combinations group.
The main effects were considered statistically significant at the 0.05 α level. All analyses were performed using Stata 15.0 (StataCorp LP, College Station, TX, USA).
RESULTS
CHARACTERISTICS OF THE PARTICIPANTS
To investigate potential systematic bias, we compared the current study sample (n = 108) with the remaining individuals of the MAS-Lactancia cohort (n = 872), excluded from this analysis as Hb data were not available in the medical files. When comparing socio-demographic variables, we did not find any differences; however, we found differences at baseline, where women in the analytical sample had an initial BMI lower than the rest of the cohort (25.5 ± 3.7 vs 26.1 ± 4.1, p < 0.05), and reported consumption of fewer supplements (55 % vs 73 %). Finally, at birth, we did not find any difference in offspring variables: weight, length, or gestational age (Table II).
Table II. Comparison of analytical sample vs. the rest of the cohort.

*Indicates proportions. p-values obtained from T-test or Fisher exact test.
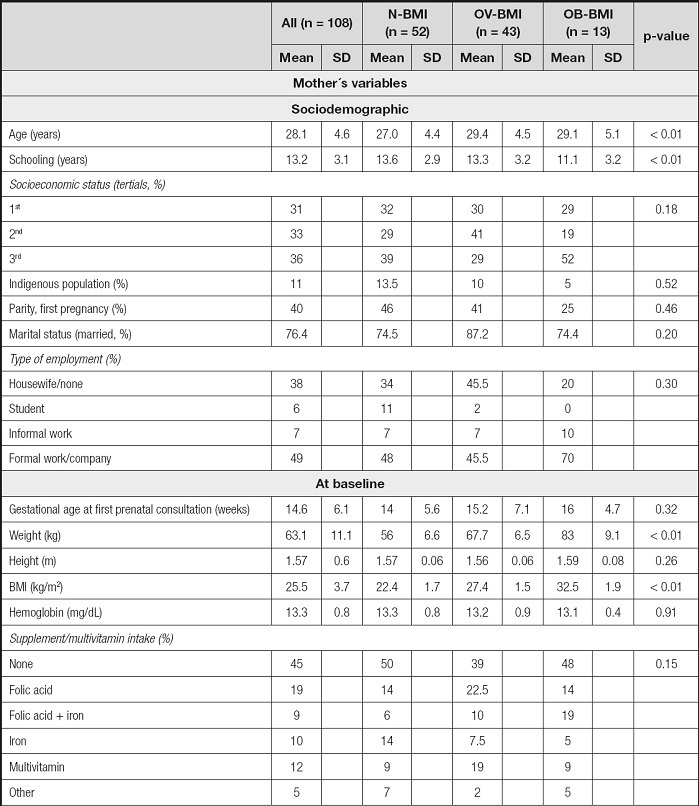
The participants´ mean age was 28.1 years (SD 4.6, range 19 to 40). Average prenatal visits were 2 (range 1-5) at IMSS clinics. Pre-pregnancy, 48 % were N-BMI, and more than half had an initial BMI ≥ 25 kg/m2 (40 % had OV-BMI, and 12 % had OB-BMI). Only one participant had a BMI < 18.5 kg/m2, i.e. underweight, and she was included in the N-BMI group (BMI 17.4 kg/m2).
Table III shows the sample main characteristics at baseline, delivery and post-partum stratified according to the pre-pregnancy BMI group. N-BMI participants were 2 years younger and had on average 2.5 years more of education compared to the pre-pregnancy OB-BMI participants (p < 0.01). Regarding socioeconomic status, indigenous identity, parity, marital status, and employment type we did not find any difference between participants in the pre-pregnancy BMI groups. In total, 55 % of participants reported consumption of vitamin-supplement or multivitamins during pregnancy, including folic acid (19 %), iron (10 %), iron plus folic acid (9 %), or others (17 %) with no difference between groups (p = 0.15). At the end of pregnancy, there was a difference in GWG according to pre-pregnancy BMI status, with a higher proportion of excessive weight gain in those with pre-pregnancy OV-BMI and OB-BMI, and a higher proportion of insufficient GWG in those with N-BMI (p < 0.01).
MAIN FINDINGS AND ASSOCIATIONS
Overall, the mean Hb level at the beginning of pregnancy (before week 14) was 13.3 g/dL (SD 0.8) with no difference between BMI-groups (p = 0.90), and none presented with anemia. In contrast, in the third trimester (after week 28), 22.8 % presented with this condition, as mean Hb levels decreased 1.5 g/dL from 2nd to 3rd trimester of pregnancy with no differences between BMI-groups (p = 0.48).
In table IV, the stratified models by pre-pregnancy BMI status are presented, and we found that Hb levels varied according to the pre-pregnancy BMI status and GWG. Hb levels at the end of pregnancy were significantly lower in those with pre-pregnancy OB-BMI and excessive GWG (12.1 g/dL, 95 % CI: 10.7-13.5) compared to those with pre-pregnancy OB-BMI and insufficient GWG (13.3 g/dL, 95 % CI: 11.9-14.8) (p = 0.04). For participants with pre-pregnancy N-BMI and OV-BMI, there were no differences in Hb levels at the end of pregnancy.
Table IV. Predictive margins and 95 % CI of the linear prediction of hemoglobin levels (n = 108).

Model adjusted by (used for margins estimations): mother´s age (mean 28.1 years), mother´s education (mean 13.3 years), parity (first pregnancy), reported supplement intake (yes, no) and gestational age (28 weeks). Italics indicates significant differences within BMI groups (insufficient GWG).
The 37.5 % were OB-BMI, 53.5 % OV-BMI, and 9 % remain within N-BMI at the end of pregnancy. Within the possible combinations of BMI classification and anemia at the end of pregnancy, 6 % had N-BMI and normal Hb levels, 3 % had N-BMI and anemia, 41 % were OV-BMI with normal Hb levels, 12.5 % OV-BMI and anemia, 26.5 % OB-BMI with normal Hb levels, and 11 % presented OB-BMI and anemia. Using these combinations, we did not find any difference at birth regarding the delivery method (p = 0.79), but regarding the gestational age classification at birth we observed that moderately preterm (32-36 weeks) and late term (41 weeks) was predominant in those mothers identified as OV-BMI, OB-BMI, and OB-BMI with anemia (Fig. 2).

Figure 2. Classification of Gestational age at delivery (%) according to the combined BMI and anemia status at the end of pregnancy (American College of Obstetrics and Gynecologists definition: moderately preterm [32-36 weeks], early term [37-38 weeks], full term [39-40 weeks], late term [41 weeks]).
When we looked at the growth trajectories (birth to 3 months of age), we noticed that infants from mothers within the same BMI classification at the end of pregnancy tended to grow differently if they also presented with anemia at the end of pregnancy. Specifically, those participants with OV-BMI and normal Hb levels at the end of pregnancy had children with higher scores in WAZ and WLZ. Also, those with OB-BMI and normal Hb levels at the end of pregnancy had children with higher scores in WLZ and triceps skinfold (Table V). The mentioned growth patterns (with higher WAZ and WLZ) were not seen in those children whose mothers presented with OV-BMI or OB-BMI with anemia.
Table V. Association of the combination of BMI status and anemia (Hb < 11 g/dL) at the end of pregnancy with growth indicators and triceps skin fold in offspring from birth to 3 months of age.

Italics indicate p-values < 0.05 in the regression models of the offspring parameters (reference N-BMI at the end of pregnancy + no-anemia).
DISCUSSION
We documented that more than 50 % of women started pregnancy with a BMI above 25 kg/m2 (40 % overweight and 12 % obese), and we observed that only 25 % of the pregnant women sample achieved an adequate GWG. In the case of those women who started pregnancy with a BMI > 25 kg/m2, more than 20 % accumulated excessive GWG. Within these two characteristics, pre-pregnancy BMI status and GWG, at the end of pregnancy we estimated that the mean Hb levels were 1.5 mg/dL lower for pre-pregnancy OB-BMI women with excessive GWG compared to those with pre-pregnancy OB-BMI women with insufficient GWG (12.1 g/dL vs 13.3 g/dL, p = 0.04).
Importantly, there is a normal reduction of Hb during pregnancy due to plasma volume expansion at the end of 1st trimester; based on a review, Wawer et al. suggested that pre-pregnancy obesity and overweight carry a greater risk of anemia (iron deficiency) for the mother (29). Some epidemiological studies have documented that overweight women have an increased risk of low iron status and higher hepcidin levels and maternal inflammation (15,30). Furthermore, a study of two cohorts in Indonesia and Ghana showed that higher BMI in early pregnancy was positively associated with Hb and reduced risk of anemia, but in that study the GWG was not considered in the models, and women had lower mean pre-pregnancy BMI than in our sample (31).
The possible explanations for why excessive GWG in pregnant women with pre-pregnancy obesity can reduce Hb levels are 1) poor dietary quality, diets restricted in total iron or animal-source iron, and high density-caloric diets, common in the Mexican population (32); 2) increased iron requirements during pregnancy, or impaired iron absorption related to higher hepcidin levels resulting from obesity-related chronic low-grade inflammation (8,13,33).
We also showed that women can end the pregnancy without anemia and with an N-BMI (6 %), OV-BMI (41 %), or OB-BMI (26.5 %); but also, with anemia with N-BMI (3 %), OV-BMI (12.5 %), or OB-BMI (11 %), which means than one of every 10 pregnant women presented obesity (BMI ≥ 30 kg/m2) and anemia (Hb < 11g/L) at delivery. This agrees with the combined prevalence of overweight and anemia in women of reproductive age reported by The Mexican National Health and Nutrition Survey (19).
Regarding offspring outcomes at birth, we did not find a significant difference in weight and length according to the combinations of mother´s BMI and anemia. This result is similar to the findings in the cohorts from Indonesia and Ghana where adverse outcomes at delivery were not found (31). However, when we explored in the longitudinal model (birth to 3 months old) in offspring´s growth trajectories, we found a statistical difference in the weight for age Z score, which was greater in offspring from women with OV-BMI and normal Hb levels. Overweight and mothers living with obesity and normal Hb levels had offspring with higher Z scores of weight for age, weight for length and triceps skinfold from birth up to 3 months old. All of these parameters are related to a higher risk of obesity and cardiometabolic alterations in childhood as have been shown in previous cohorts (34, 35), including a Mexican cohort from the same socioeconomic strata (36).
When constructing the database, we observed that only 11 % of the revised medical files had recorded Hb levels in the antenatal visits; these data are relevant as we expected to have this information for all pregnant women at least once. The number of antenatal visits was on average surprisingly low at 2 (range 1 to 5), which also reflects that most of the pregnant women included in this report did not meet the WHO recommended minimum of 4 and optimally 8 antenatal visits. This also has to be seen in the light of the fact that the vast majority of pregnant women in Mexico (98.7 %) attend antenatal care, and they are in contact with the healthcare system (21), but this contact is not always during the first weeks of pregnancy, when the prenatal programs are supposed to be initiated as folic acid and iron supplementation.
STRENGTHS AND LIMITATIONS
The main strength of the study is the longitudinal design, with repeated measures during pregnancy and after birth, which allowed us to establish temporality in the reported associations. A limitation of the study is that we did not measure ferritin or iron stores, but only Hb as the parameter commonly measured in IMSS clinics; however, it has been documented that > 50 % of anemia in pregnancy is due to iron deficiency (37). Furthermore, pre-pregnancy BMI was self-reported, which can produce an error in the estimations; nevertheless, a previous study has documented that it can explain 88 % of the variance in estimated pre-gravidae weight (38), and it can produce an underestimation of overweight and obesity (39), which means that our results could be stronger with an objective measure. Finally, the relatively small sample size limits the power of our analysis, but when compared to the rest of the cohort, we observed no statistically significant differences, except for one variable, reflecting internal validity.
IMPLICATIONS FOR THE HEALTH SYSTEM
These results should be taken into consideration at prenatal care, especially when the current health system is facing larger numbers of overweight or obese pregnant women. Identifying women not only with overweight/obesity but also with its combination with anemia represents a new challenge to health services and an increasing problem, as approximately 1 of every 10 pregnant women could probably have both conditions in pregnancy. It is well established that both maternal over- and under-nutrition, including micronutrient deficiencies leading to anemia, increase the risk of non-communicable disease in the offspring via fetal programming (40).
As women are often highly motivated for behavioral changes, especially when associated with benefits to their offspring, consequently, intervening at this stage of the life-course has the potential of a significant impact on the future health of both mother and her offspring.
CONCLUSION
Our results suggest that excessive GWG is associated with having lower Hb levels at the end of pregnancy among women living with obesity. Additionally, newborns presented higher scores in weight for age, weight for length, and triceps skinfold from birth to 3 months of age, parameters related to childhood obesity, when mothers presented overweight and obesity only, but not in those mothers with overweight or obesity and with anemia.