Introduction
A large part of the stress research in humans has focused on the effects of psychosocial stress on the neuroendocrine response, which has been related to important consequences in psychopathology and physical illness. In many experimental studies, stress has been induced by exposing the participants to aversive sensory stimuli or psychological experiences, including demanding cognitive tasks and social evaluation. Particularly, in relation to psychosocial stress, many studies have tried to employ laboratory situations that are similar to real life stressors (Moya & Salvador, 2001). Public-speaking tasks have been shown to be a potent social stressor with a strong impact on endocrine and cardiovascular responses (Dickerson et al., 2004). Among these protocols, the Trier Social Stress Test (TSST) has been widely used in psychobiological stress research because it has been found to evoke robust endocrine and cardiovascular responses in the majority of the participants (Kirschbaum et al., 1993).
The TSST mainly consists of a short mock job interview and a mental arithmetic task in front of an audience of two or three people. Thus, it includes the two main factors for robust hypothalamic-pituitary-adrenal (HPA) axis activation: social evaluative-threat and uncontrollability. As long as the protocol comprises both tasks in front of evaluative judges, most participants respond with a significant increase in free salivary cortisol, resulting in an overall average two-fold increase over baseline (Dickerson and Kemeny, 2004). Salivary cortisol, the main product of the HPA axis, is one of the most useful markers of the stress response to the TSST (Allen et al., 2017). Furthermore, the TSST increases anxiety (Rimmele et al., 2009) and worsens negative mood (Yim et al., 2010).
Although the TSST is a highly controlled procedure, and committee members are usually well-trained prior to an experiment, some nuances in the interactions between a specific participant and committee member cannot be completely controlled and may introduce some variability into the data. In addition, depending on the requirements of a particular study, different adaptations are made that can help to overcome some of the limitations.
Thus, innovative adaptations of the original TSST have been made that have relative advantages. Some different TSST versions have been employed, some of which have also been validated. For instance, the TSST for groups (TSST-G), which increases the number of study participants, has been shown to produce a significant HPA axis and autonomic nervous system (ANS) response (Allen et al., 2014; Boesch et al., 2014; von Dawans et al., 2011). The heart rate increases throughout the TSST-G and returns to the pre-stress level a few minutes after the stressor ceases (Childs et al., 2006), replicating the underlying adrenaline increases after the original TSST (Gold et al., 2004). Moreover, a virtual reality version of the TSST (TSST-VR) has been designed (Kotlyar et al., 2008) that removes any variability due to interactions between participants and committee members. All these adaptations and variations have been made in the context of the TSST's application. So far, to our knowledge, no one has modified the participants' exposure conditions. We wonder whether changing the participants' exposure situation will influence their response to stress.
Because TSST protocol modifications may influence stress responses by changing the perception of stressfulness, the aim of this study was to measure the hormonal, autonomic, and affective responses to a modified version of the TSST stressor where participants were seated while performing the TSST instead of standing, the arithmetic task was shortened from 5 to 3 min, and there was only one person on the jury. Due to the relative lack of knowledge and consistency regarding the importance of sex/gender and the menstrual cycle phase in autonomic nervous system (ANS) markers of the stress response, the current study also aimed to analyze how sex and the menstrual cycle phase affect the ANS response to stress. To address these aims, we analyzed the heart rate (HR) and electrodermal activity (EDA) as indicators of ANS activity and the salivary cortisol response. In addition, because personality traits, specifically trait anxiety, can play an important role in the psychophysiological stress response (Chida & Hamer, 2008), in order to control individual differences, a measure of trait and state anxiety was included. Based on previous literature, we expected hormonal and sympathetic activation in response to the TSST, measured through HR, EDA, and cortisol. In relation to the importance of sex differences and the menstrual cycle phase in the response to stress, we aimed to explore the profiles of stress responses in women in different phases of the menstrual cycle, compared to men.
Method
Participants
Thirty-eight young university students (11 men and 27 women) from 18 to 25 years old (M = 19.21; SD = 1.80) participated in this study. All of them gave their written informed consent to participate in the study. The participants were selected by a General Health Questionnaire that included the menstrual history and recent changes in menstruation in the sample of women. All were volunteers, unmarried, and had no physical or psychological problems. The women were nulliparous, reported having had regular menstrual cycles during the last three menstruations (23-35 days), and had no history of gynaecological disorders. None of the women had taken oral contraceptives or medication that affected their neuroendocrine system. Participants were not informed about the task to be performed until the experimental session began. Each of them participated in a single experimental session that lasted approximately 50-60 min.
Determination of the phases of the menstrual cycle
The women were distributed into two groups, depending on the menstrual cycle phase (16 follicular and 11 luteal). Two different estimation procedures were employed to determine the hormonal phase of each woman. First, all the cycles were converted to a standard 28-day cycle (Rossi and Rossi, 1980). The day of the onset of the last menstruation and the real length of the studied cycle were taken as points of reference. Based on the results of this procedure, the women were invited to attend the experimental sessions. Second, during two complete menstrual cycles, the sublingual Basal Body Temperature (BBT) of each woman was recorded daily on a graph for 5 min before getting up to estimate the ovulation point. The ovulation point is identified as the day before the temperature rises. To analyze the BBT, the “smoothed curve” (SMC) method was used, as described by McCarthy and Rockette (1983, 1986).
Along with the instructions to participate in the study, the selected women received an oral thermometer and a temperature graph. The women were trained in the graphic registration of their basal body temperature (TBC), and they were told what day and time they should visit the laboratory. Each woman went to the laboratory twice, once corresponding to the selected phase of the menstrual cycle and a second time to confirm the evaluated menstrual cycle phase.
Apparatus
The PowerLab /8SP data acquisition system, with a voltage range of ± 10 V, controlled by an internal microprocessor 68340 of 32 bits at 16 MHz and a maximum sample rate of 20 KHz on eight channels, was employed for the acquisition, amplification, and filtering of the EDA and HR signals. This system converted analogical signals to digital by means of a 16-bit A/D converter. The recording unit was connected to a PC through a USB port (500 KB/s, maximum data transfer rate). The control of the acquisition system, the parameter registration, and the data storage was carried out by Chart v5.0 for Windows of AD Instrument, which analysed the response quantification. All the psychophysiological signals were sampled continuously at 1000 Hz throughout the experimental session.
A Technics RS-B40 tape-recorder was used to record and deliver the instructions. Cardiac activity was recorded by the software Chart v5.0 for Windows of AD Instruments through an electrocardiogram (EKG), providing a pondered mean of Heart Rate (HR), beat to beat.
Electrodermal activity (EDA) was recorded by a constant voltage of .5 V through bipolar placement of 7-mm Ag/AgCl standard surface electrodes, attached by adhesive collars to the thenar and hypothenar eminences of the non-dominant hand. Electrodes were filled with .068-M NaCl Unibase electrode paste (Fowles et al., 1981), which was used as the contact medium. The raw signal was acquired with a Biosig-CP1 module (Cibertec, Madrid, Spain) and calibrated to detect activity in the 0-100 µSiemens (µS). This module provided a double output in order to obtain the skin conductance levels (SCL) and the frequency of non-specific skin conductance responses (NS-SCRs, with AC coupling) in two separate channels.
Measures
Autonomic measures
Heart Rate (HR) and Electrodermal Activity (EDA) were measured continuously. In the EDA, the frequency of non-specific skin conductance responses (NS-SCRs) and skin conductance levels (SCL) were registered. HR in beats per minute was extrapolated from EKG data by Acqknowledge software. NS-SCRs were obtained with two Ag/AgCl electrodes (TSD103A) with a contact area of 6 mm in diameter located on the middle phalanxes of the fore and index fingers of the non-dominant hand by means of adhesive collars. Hypoallergenic gel (G100) was used as the contact medium between skin and electrode. A skin conductance module (GSR100A) amplified the electrical signal through a circuit of constant voltage (.5 V).
Salivary cortisol
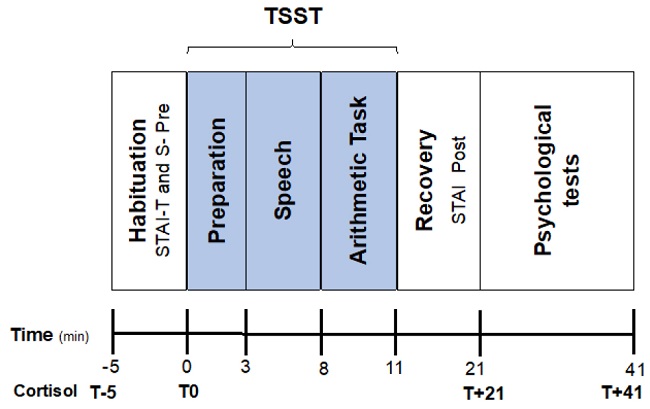
Four saliva samples were collected for the assessment of free salivary cortisol: five min before the onset of the experimental task (t-5), immediately before the preparation phase (t0), after the recuperation period (t+21), and 20 minutes after the cessation of the laboratory stressor (t+41). Participants received instructions for collecting the salivary sample at the beginning of the study. Saliva was collected using Salivettes (Sarstedt, Rommeelsdorf, Germany) that were frozen at -80ºC until determination. All the samples of each participant were analysed in duplicate in the same assay. Hormonal determinations were made by RIA (radioimmunoassay) in the Research Support Service (RSS) at the University of Murcia. Salivary cortisol was determined by a commercial kit adapted to salivary levels, as recommended in the protocol (Orion Diagnostic, Espoo, Finland). Cortisol levels were expressed in nanomoles per liter.
Subjective measures
The State-Trait Anxiety Inventory (STAI) (Spielberger Gorsuch and Lushene, 1970) was employed to measure anxiety. It is a 40-item self-report scale that measures two types of anxiety dimensions: state anxiety (20 items), a temporal anxiety or a feeling of tension, apprehension, nervousness, and activation (arousal) of the ANS related to an event; and trait anxiety (20 items), a relatively stable individual difference in anxiety proneness. Each item presented a description, and respondents were asked to indicate the extent to which each item described them on a 4-point scale.
All participants completed the STAI-S (state anxiety) before and after the task, and the STAI-T (trait anxiety) only before the task. The Spanish version of the scale had a Cronbach's alpha ranging from .90 to .93 (Seisdedos, 1988).
Stress task
The TSST (Kirschbaum et al, 1993) was employed as a psychosocial stressor, but with some variations. To register the autonomic activity, we employed a version that consists of a preparation phase (3 min), followed by free speech (5min) and a mental arithmetic task (3 min) in front of a female or male evaluator (counterbalanced) who was a university teacher not familiar to the students. The entire task procedure takes about 11-12 min. The variations compared to the original version were that the participant, instead of standing in front of the evaluator, was seated, and instead of two evaluators, there was only one. In addition, the preparation phase was reduced from 10 min to 3 minutes, and the arithmetic task was also shortened from 5 to 3 minutes.
Procedure
Upon arrival to the laboratory, participants were placed in a first room (room A) where they filled in the anxiety inventory (STAI-S and T) and supplied the first saliva sample. After washing their hands with soap and water, participants were seated in a comfortable armchair in an adjacent room (room B), located inside a soundproofed experimental booth (3.06 x 2.02 x 2.36 m). At all times, the participants could communicate with the experimenter through a system of intercoms connected to the exterior of the booth. This booth was maintained at a temperature of 21.77 (± 2º C.) and humidity of 64.98% (± 2 %.). The light was kept constant during the entire experimental session. After being seated in the armchair and having the electrodes placed on them, participants were given the instructions for the experimental session, which had previously been recorded by a feminine voice.
Each experimental session lasted about 50 minutes and consisted of the following periods: the habituation phase, which was used as baseline (5 min), where the participant completed the pre-task STAI. Next, each participant was directed to room B and received brief instructions about the task to be performed (“You have 3 min to prepare a 5 min speech to convince the evaluator, who will be in front of you, that you are ready to work as a psychologist. Your speech will be evaluated by a professor”). During this preparation phase (3 min), each participant provided the second salivary sample and thought about the speech. Then, the evaluator went into the experimental room (B), sat in front of the participant, and told him/her to start (speech task, 5 min). The evaluator took notes during the speeches, but without speaking or making any facial expressions. After the arithmetic task (3 min), this phase was the same as in the original version, but shorter (3 min vs 5 min). Afterwards, the evaluator left the room, and the participants stayed another 10 min without any stimulation (recovery phase). Finally, the participant completed the post-task STAI. After these 10 min, the third saliva sample was obtained. During the entire experimental session, autonomic activity was continuously recorded. After the experimental session was over, the participants returned to room A, where they spent 20 minutes completing other psychological tests (not included in this paper) and provided the last salivary sample. The experimenter was always female. The session took place in the Laboratory of Psychophysiology at the University of Murcia from Monday to Friday between 9 and 14 hours. The sequence is presented schematically in Figure 1.
Statistical analyses
Cortisol levels and autonomic measures were tested for normal distribution and homogeneity of variance using the Kolmogorov-Smirnov test. These analyses did not reveal significant deviations from normality. Therefore, all subsequent analyses and the values represented in the figures are absolute values.
Autonomic measures were analysed using analysis of variance (ANOVAs) for repeated measures (5x3), with “Time” (Habituation, preparation, speech, arithmetic task, and Recovery) as the within-subjects factor and “Group” (/luteal/ follicular /men) as the between-subjects factor.
Cortisol measures were analysed using analysis of variance (ANOVAs) for repeated measures (4x3), with “Time” (t-5, t0, t+21, t+41) as the within-subjects factor and “Group” (/luteal/ follicular /men) as the between-subjects factor.
State-anxiety (pre-/post-task) was analysed using analysis of variance (ANOVAs) for repeated measures (2x3), with “Time” (pre vs post-task) as the within-subjects factor and “Group” as the between-subjects factor. Trait-anxiety (only pre-task) was analysed using one-factor ANOVAs, with “Group” as the between-subjects factor.
All statistical analyses were performed with SPSS v21.0 for Windows, and the alpha level for all comparisons was set at p ≤ .05.
Results
Demographic and anthropometric variables
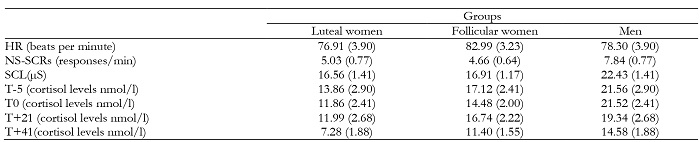
The main demographic and anthropometric characteristics of the groups are shown in Table 1. No significant differences were found between groups on Age, BMI, or scores on the STAI-T.
Electrophysiological measures
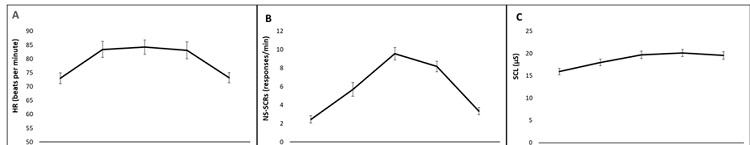
The ANOVAs showed significant effects of the “Time” factor for HR (F (4, 140) = 15.54, p ≤ .001; η2 p = .30), for NS-SCR (F (4, 140) = 50.51, p ≤ .001; η2 p = .59) and SCL (F (4, 140) = 64.86, p ≤ .001; η2 p = .65). In relation to the “Group” factor, significant effects were found for NS-SCR (F (2, 35) = 5.50, p = .008; η2 p = .23) and SCL (F (2, 35) = 5.729, p = .007; η2 p = .24). No significant effects of the “Time x Group” interaction were found.
Post-hoc analyses showed the highest HR, the highest number of NS-SCRs during the speech phase, and the highest level of conductance on the arithmetic task, with both phases showing significant differences compared to the habituation and recovery phases (for all p ≤ .05). In addition, men showed a higher frequency of NS-SCR and greater SCL than the two groups of women, whereas non-significant differences were found between the two female groups: for NS-SCR (Men vs Luteal, p = .04; Men vs Follicular, p = .01; Luteal vs Follicular, p = 1.00); for SCL (Men vs Luteal, p = .017; Men vs Follicular, p = .014; Luteal vs Follicular p = 1.00). (See Tables 2 and 3 and Figure 2).
Table 2: Means and standard error of the mean (S.E.M.) in parentheses for the significant factor “time” in non-specific skin conductance responses (NS-SCRs), skin conductance levels (SCL), and heart rate (HR) for the total sample.

Table 3: Means and standard error of the mean (S.E.M.) in parentheses for the groups on autonomic and hormonal measures.
Hormonal measures
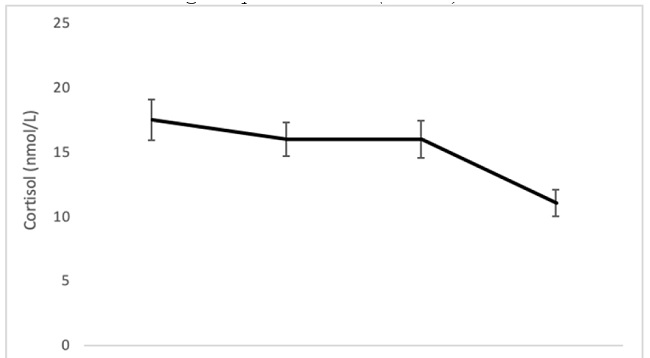
Significant effects of “Time” (F (3, 105) = 9.19, p ≤ .001; η2 p = .20) and “Group” (F (2, 35) = 3.90, p = .029; η2 p = .18), but not of the “Time x Group” interaction, were found. Regarding the “Time” factor, post hoc analyses showed that the T+41 sample was significantly different from the rest of the samples, presenting the lowest levels (T-5 = 20.35; T0 = 18.23; T+21 = 16.83; T+41 = 11.09) (for all comparison, p ≤ .001). (See Figure 3).
In relation to the “Group” factor, men showed higher cortisol levels only in comparison with the women in luteal phase (p = .025). No significant differences were found between the two groups of women (p = .51). (Luteal = 11.25; Follicular = 14.94; Men = 19.25).
State anxiety
Results for state anxiety did not show any significant effects of the factors “Time” (F (1, 35) = 1.178, p = .285; η2 p = 0.03), “Group” (F (2, 35) = 2.343, p = .111; η2 p = 0.11), or the “Time*Group” interaction (F (2, 35) = 1.088, p = .348; η2 p = 0.05). (Luteal pre = 21.63 - post = 20.00; follicular pre = 21.93 - post = 22.93; men pre = 19.09; post = 14.72).
Discussion
The present study compared the hormonal, autonomic, and affective responses to a modified version of the TSST in men and women in different phases of the menstrual cycle. The results indicated that the speech phase of the TSST was the most efficient in eliciting enhanced autonomic responses in the measure of electrodermal activity in comparison with the values of the other phases. However, this effect was not found in the heart rate, where the TSST did not produce significant changes compared to the rest or recovery phase. The high variability in HR both within and between participants during the laboratory challenge contributes to the fact that it often fails to be a sensitive index of the objective stress response (Quintana and Heathers, 2014). Therefore, these results would partly support findings from other studies that showed marked increases in the physiological response during the TSST (Kirschbaum et al., 1993; Nater et al., 2005; Espin et al., 2019).
However, the TSST had a low impact on the salivary cortisol response and the psychological state. Our results show that the cortisol response to the TSST and state anxiety, contrary to expectations, decreased instead of increasing. One explanation for these results is that the extent to which a stressor triggers an elevation in cortisol is dependent on a variety of factors, including novelty, uncertainty, and negative emotions (Dickerson and Kemeny, 2004). Thus, it will be necessary to analyse whether the modifications made in the TSST protocol can explain these results.
In our study, the TSST protocol was modified slightly in order to register the electrophysiological measures. The TSST task was stress-producing because it induced increases in the vegetative activity, but it may not have been sufficient to provoke changes in hormonal and affective responses. Reduced responsiveness to a stress stimulus may be the result of an inability to respond with an adequate hormone release, or it may be due to a decreased perception of the stimulus. Where our results are concerned, the latter might be the case because state anxiety following stress exposure decreased instead of increasing in all the participants.
Moreover, methodological factors could have influenced the lack of HPA axis response to the TSST, for example, the time of day or reducing the preparation period to 3 min. However, a recent meta-analytical assessment of the effects of protocol variations on cortisol responses to the Trier Social Stress Test showed that the time of day when the TSST is conducted does not significantly influence the overall cortisol response (Goodman et al., 2017). These same authors suggest that reducing the preparation period even to 3 min does not negatively affect the strength of the cortisol stress response. Unlike frequent variations in the speech preparation time, very few studies have varied the length of the verbal or arithmetic components. In other words, the majority of the studies followed the original instructions and obtained robust results, suggesting that theoretical job interviews and arithmetic tasks are appropriate and effective stressors. Therefore, it seems advisable to maintain the original time of both the speech and arithmetic tasks (Goodman et al., 2017). This meta-analysis also assessed studies that compared the cortisol stress response in TSST studies that used three-member panels with those that used two-member panels. The authors suggest that three members may be slightly more effective. In our case, we had only one person on the panel.
Therefore, taking into account the variations we made in the TSST protocol, we can say that reducing the time of the arithmetic task, using only one person as a jury, and seating the participant comfortably in front of the evaluator could have influenced the lack of cortisol response to the TSST found in our participants.
Sex seems to be a fundamental factor when studying the response to stress because a large amount of research has been published on sex differences in autonomic nervous system (ANS) and hypothalamic-pituitary-adrenal axis (HPA) responses to stress, with inconsistent results. Differences have been found between men and women on several baseline or pre-stress cardiovascular measures, such as HR and blood pressure (Suarez et al., 2004), as well as effects of the hormonal status on the physiological response to acute stress (Stark et al., 2006; Wolf et al., 2001). Although there are exceptions, between puberty and menopause, HPA and autonomic responses tend to be lower in women than in men of the same age (Kirschbaum et al., 1999; Kajantie and Phillip., 2006).
In our study, we did not find sex differences or effects of the menstrual cycle phase in the autonomic and cortisol response to the TSST, but we found a higher autonomic response to the experimental session in men in comparison with both groups of women and a greater cortisol response in men than in women in the luteal phase. Some authors have suggested that estrogens have a diminishing effect on sympathetic activity (Kajantie & Phillips, 2006), which could explain the lower autonomic activity found in both groups of women compared to men. Our results would confirm the findings of several studies that have reported no sex differences in cardiovascular responses to the TSST (Kirschbaum et al., 1999; Kelly, et al., 2008; Villada, et al, 2018).
The higher levels of cortisol found in men compared to women in the luteal phase is consistent with the findings of other studies where a higher salivary cortisol response has been found in men than in women (Hidalgo et al., 2014; Kirschbaum et al., 1999; Stephens et al., 2016; Uhart et al., 2006). However, it should be noted that, in several studies, women in the early follicular phase and women using hormonal contraception have been included because they usually show responses to stress that differ more than those of women in the luteal phase when compared to responses of men. Therefore, it is likely that the null effects found in our study were due to the finding that the magnitude of the cortisol response in the majority of our sample (i.e., more women in their luteal phase than men) was low. Therefore, the role of sex hormones is an issue that needs to be considered to better explain why some studies show no sex differences in stress responsiveness, and why, when differences are found, the results are inconsistent.
Any recommendations based on our findings have to be considered in light of several limitations. A limitation of this study is our small sample size; thus, the present study should be considered a pilot study, and the results should be interpreted with caution and replicated with larger sample sizes. Another limitation is the fact that the sex hormones were not measured. Although we used a rigorous method to estimate the menstrual cycle phase and check the ovulatory phase, further studies should include the concentrations of women's sex hormones in each menstrual cycle phase evaluated.
In summary, the TSST has been shown to be a good psychosocial stressor, but when some aspects of the procedure are changed, the task is not efficacious enough to provoke an enhanced HPA activity or affective state. We can state that the TSST has limited applicability because studying certain electrophysiological measures, such as AED or EKG, requires participants to be in other conditions where the TSST does not yield consistent results. More studies are needed that take these aspects into account.
Nevertheless, the present study is the first to confirm that a seated version of the TSST did not provoke the expected response in the participants in terms of an increase in the HPA axis activity and a worsening of their affective state.