My SciELO
Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
The European Journal of Psychiatry
Print version ISSN 0213-6163
Eur. J. Psychiat. vol.22 n.3 Zaragoza Jul./Sep. 2008
Minor physical anomalies in Tourette syndrome
Györgyi Csábi MD, PhD*; Júlia Gádoros MD, PhD**; Sára Jeges PhD***; Eszter Gyenge MD****; Mátyás Trixler MD, PhDDSc*****; Tamás Tényi MD, PhD*****
* Department of Pediatrics, University of Pécs, Faculty of Medicine, Pécs
** Vadaskert Child and Adolescent Psychiatry Centre, Budapest
*** Central Research Laboratory, University of Pécs, Faculty of Medicine, Pécs
**** Outpatient Centre for Child Psychiatry, Pécs
***** Department of Psychiatry and Psychotherapy, University of Pécs, Faculty of Medicine, Pécs. Hungary
ABSTRACT
Background and Objectives: The prevalence of minor physical anomalies (prenatal errors of morphogenesis) was evaluated in patients with Tourette syndrome to get indirect data on the possible role of aberrant neurodevelopment in the aetiology of Tourette syndrome. No published study is known on the minor physical anomaly prevalence in this recently intensively investigated disorder, and connecting to current opinions on a possible role of aberrant neurodevelopment in Tourette syndrome it seems important to introduce trait marker research focusing on brain maldevelopment.
Methods: A scale developed by Méhes1,2 was used to detect the presence or absence of 57 minor physical anomalies in 24 patients with Tourette syndrome and in 24 matched controls 21 boys and 3 girls were evaluated, the age of onset of illness among the Tourette patients was between the age of 5 and 13.
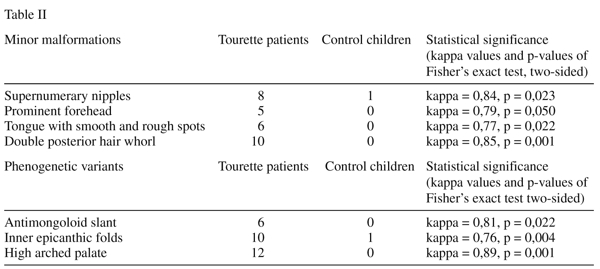
Results: The mean value of all minor physical anomalies was significantly higher among the group of patients compared with controls. (Mann - Whitney U - value: 49, 50, -Z = - 4,92, p = 0,001) In the case of 7 minor physical anomalies we could demonstrate statistically significant differences between the Tourette and the control sample. In the case of 4 minor malformations (supernumary nipples, prominent forehead, tongue with smooth and rough spots, double posterior hair whorl) and of 3 phenogenetic variants (antimongoloid slant, inner epicanthic folds, high arched palate) a significantly higher frequency was observed compared with control individuals. However after Bonferroni correction for the Fisher’s Exact test, only double posterior hair whorl and high arched palate showed a significantly higher frequency compared to control children (p = 0.001).
Conclusions: The overrepresentation of minor physical anomalies in Tourette syndrome can strongly support the view that this disorder is related to pathological factors operating early in development.
Key words: Minor physical anomalies, Tourette syndrome, Neurodevelopment.
Introduction
Tourette syndrome is a neuropsychiatric syndrome with onset in childhood that is characterized by multiple chronic tics. The syndrome is characterized by involuntary rapid, non-rhythmic skeletal movements and sounds. Simple vocal tics include throat clearing, whistling, snorting, barking, growling, whereas complex vocal tics consist of words and phrases sometimes with obscene or aggressive content. Simple motor tics can be observed in the form of eye blinking, grimaces and jerks, whereas complex motor tics involve stereotyped facial expression, grooming, touching, hopping, banging and so on3. Conclusive evidence of the pathophysiological basis of tic disorders is lacking, but converging data support the notion that Tourette syndrome is a genetic disorder involving abnormal dopaminergic-excitatory amino acid interactions in neural circuits bridging parts of frontal cortex, basal ganglia and the thalamus. Both structural and functional neuroimaging studies have contributed to the understanding the aetiology of the syndrome. Studies using structural magnetic resonance imaging found decreased volume of the left basal ganglia4-6, while other studies reported abnormal size of the corpus callosum and enlarged right lateral ventricle7,8. As in other disorders (autism, attention-deficit hyperactivity disorder, dyslexia, schizophrenia, bipolar affective disorder, obsessive-compulsive disorder) from the neurodevelopmental spectrum9,10, results from structural neuroimaging studies can get a parallel support by investigations on the phenotypical marker profile.
Minor physical anomalies or informative morphogenetic variants are mild, clinically and cosmetically insignificant errors of morphogenesis which have a prenatal origin and may bear major informational value for diagnostic, prognostic and epidemiological purposes11. The presence of minor physical anomalies is a sensitive physical indicator of embryonic development. They are of value to the clinical researchers as they serve as indicators of altered morphogenesis that occured early in gestation. Since both the central nervous system and the skin derived from the same ectodermal tissue in utero, minor physical anomalies may be external markers of abnormal brain development. Minor physical anomalies are considered to develop during the first and/or early second trimester of gestation12,13 and represent potentially valuable indices of disturbances in early neurodevelopment. Once formed they persist into adult life and are readily detected on visual examination of the particular body area. Minor physical anomalies have been found with increased frequency in autism, hyperactivity, epilepsy, learning disabilities, speech and hearing impairments, mental retardation, poor motor coordination, attention deficit disorder, fetal alcohol syndrome, cerebral palsy11,13,14 schizophrenia15-17 and bipolar affective disorder2.
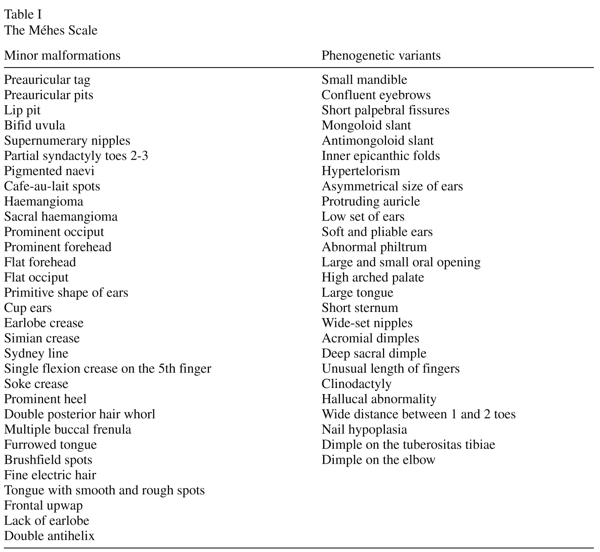
As we have discussed earlier2,18,19 differences and contradictions between studies on minor physical anomalies among adults and children with different psychiatric disorders, may be associated, partly, with the problems in the use of the Waldrop-scale for the detection of these signs. The Waldrop-scale contains only 18 minor physical anomalies20 while in recent pediatric literature more than 50 anomalies have been listed1,21,22. An other basic problem with the Waldrop-scale that it makes no distinction between minor malformations, which arise during organogenesis and phenogenetic variants, which appear after organogenesis19. Based on the report of the International Working Group23 in 1985, both Opitz24 and Méhes1 urged a clear distinction between morphogenetic events developing during and after organogenesis. Minor malformations are always abnormal and are qualitive defects of embryogenesis , which arise during organogenesis. All malformations are developmental field defects and usually they are all-or-none anomalies. In contrast phenogenetic variants are quantitative defects of final morphogenesis and arise after organogenesis. Morphologically phenogenetic variants are the exact equivalents of normal antropometric variants. Using a list of minor physical anomalies containing 57 minor signs collected by Méhes1,2,13 previously we have studied the prevalence of minor physical anomalies in patients with schizophrenia, alcohol dependence and major depression2,18,25, and recently the list and detailed definitions has become also acceptable for researchers, who wish to adapt our suggested modifications for the investigation of minor physical anomalies2.
The aim of the present study was to investigate the rate and topological profile of minor physical anomalies in a group of patients with Tourette syndrome. The following hypotheses have been tested: (1) Minor physical anomalies are more common in patients with Tourette snydrome than in normal subjects, (2) a higher rate of minor physical anomalies is found predominanlty in the head and facial regions in patients with Tourette syndrome than in normal controls. We consider that this kind of clinical morphological study can give indirect data concerning the neurodevelopmental component of the aetiology of this disorder.
Material and methods
Participants
Using a list of minor physical anomalies 57 minor signs collected by Méhes1,2,13 24 consecutively admitted patients for an outpatient evaluation or consultation because of Tourette syndrome and 24 healthy controls matched based on sex, age and ethnical origin were evaluated. Patients were recruited from outpatient clinics at Budapest and Pécs. Both departments serve for children with psychiatric problems from the general population, the age range for treated children is between 1 to 18 years. The distribution of the gender of patients has showed a stronger male predominance than it is known from epidemiological studies26, 21 boys and 3 girls were evaluated, the age of onset of illness among the Tourette patients was between the age of 5 and 13. At the time of examination the patients age was between 11 and 16 years. All patients lived with their families and attended regular schools. 13 patients received the comorbid diagnosis of obsessive-compulsive disorder and 2 children the diagnosis of Attention Deficit Hyperactivity Disorder. Children with other comorbid diagnoses (mental retardation and with any other Axis I and II diagnoses) were excluded from this study. The comparison group of children were from local elementary schools. Both parents and children gave consent, no compensation was given for participation in the study.
Methods
We have used the Méhes Scale for evaluation of minor physical anomalies, which includes 57 minor signs1,2,13. The evaluated minor physical anomalies are shown in Table I. All items in the Waldrop-scale except for head circumference and longer third toe were included in our list of minor physical anomalies. A clear differentiation between minor malformations and phenogenetic variants were introduced, the scale and detailed definitions were published earlier2.The scale is appropriate for use with both adult and pediatric patients. In all cases patients and their parents gave informed consent, the study was performed in accordance with the Declaration of Helsinki and was evaluated following institutional guidelines. Two examiners, one unaware and one aware of the diagnosis, investigated all the patients and controls separately. The raters were trained by Professor Károly Méhes, and they participated earlier in many minor anomaly studies, and they have a long clinical experience in dysmorphology. The blindness of the examiner who was unaware of the diagnosis was established as she (Gy. Csábi ) took part paralelly in many different minor physical anomaly studies (childhood schizophrenia, ADHD, mental retardation, Tourette syndrome, dyslexia, drug abuse, disruptive disorder) and she has not got any knowledge that a certain child was from an investigated or from a control group. The diagnoses of the patients were evaluated independently by two experienced child psychiatrists according to the D Diagnostic and Statistical Manual-IV27.Only those meeting the Diagnostic and Statistical Manual-IV criteria for Tourette syndrome unanimously were considered for the study. The examination of minor physical anomalies was done qualitatively (present or absent) without scores being used, but where it was possible, measurements were taken with callipers and tape to improve the objectivity of examination. Techniques and standards of measurement were borrowed from the works of Feingold and Bossert21 and Méhes1,13.
Statistics
Before the statistical analyses interrater reliability was tested and the kappa coefficient was > 0,75 for all items, so the awareness or unawareness on the diagnosis didn’t influence the results. Statistical analyses were carried out by applying the Mann- Whitney U-test for the analyses of all markers. For the analysis of the frequency of each individual minor physical anomalies the two-sided Fisher’s exact probability test was used. A Bonferroni correction was used setting the p-value for the Fisher’s Exact Test to p=0.001.
Results
We should consider as a robust finding that in the Tourette sample 12 patients had more than 5 minor physical anomalies, 4 patients had 5, 5 individuals had 3 or 4 , 3 patients had 2 anomalies and no patients were free from minor physical anomalies. In the control group no subject had more than 5 minor physical anomalies, 4 persons had 3, 10 subjects had 1 or 2 anomalies and 10 subjects were without any minor physical anomalies.
The observed frequency of minor physical anomalies for the patients and the control groups were tested by the Mann-Whitney U-test , the mean value of all signs was significantly higher among the patients group compared to controls. The values of the Tourette sample differed significantly from the control group (Mann-Whitney U - value: 49,50, -Z = - 4,92, p=0,001) Mean value in the Tourette group: 5,458, standard deviation: 2,146, standard error: 0,438. Mean value in the control group: 1,108, standard deviation: 1,178, standard error: 0,241. In the case of 7 minor physical anomalies we could demonstrate statistically significant differences between the Tourette and the control sample by the use of Fisher’s exact probability test for the analysis of the frequency of each minor physical anomalies individually. As it is shown on Table II, in the case of 4 minor malformations (supernumerary nipples, prominent forehead, tongue with smooth and rough spots, double posterior hair whorl) and of 3 phenogenetic variants (antimongoloid slant,inner epicanthic folds, high arched palate) a significantly higher frequency was observed compared to control individuals. However after Bonferroni correction setting the p-value for the Fisher’s Exact test to p=0.001, only double posterior hair whorl and high arched palate showed a significantly higher frequency compared to control children. On Table II. these two anomalies are highlighted.
Discussion
Since the available evidence indicates that minor physical anomalies arise through processes which act during the early stages of embryonic and fetal life, the overrepresentation of these anomalies in patients with Tourette syndrome can support the view that this disorder is related to factors operating early in development. Our study on the minor physical anomaly profile in Tourette patients emphasize the scientific importance of previous studies on the structural morphology among patients with this disorder4,6,8. Hyde et al.7 performed a morphometric analyses of magnetic resonance imagings of 10 monozygotic twin pairs discordant for severity of Tourette syndrome but concordant for the presence of tic disorders. In the relatively more severely affected twins they could demonstrate significantly reduced volumes of the right caudate, while the mean volume of the left lateral ventricle was 16% smaller in the more severely affected twins than the less severely affected twins. In our study, we have found a significantly higher number of anomalies in the case of 4 minor malformations, which arise during the organogenesis, and in the case of 3 phenogenetic variants which arise after organogenesis. It seems important to mention that from the 7 minor anomalies which were significantly more common among the Tourette patients, 6 involved the regions of the head suggesting a relationship with an abnormal neurodevelopmental process. Connecting to the view of Méhes1 we emphasize the essential informative importance of the significantly increased rate of supernumerary nipples and of double posterior hair whorl, as a wide range of pathological anomalies associated with supernumerary nipples has been described28,29 and that abnormal hair patterings may call attention to imparied early development of the central nervous system1,30,31.
To see as a limitation of the study, we should be cautious not to speculate from this minor physical anomaly study on the timing of possible genetic and/or epigenetic insults influencing brain development, as futher studies on different population cohorts need to clear up the minor physical anomaly profile in Tourette syndrome. Since data concerning the structural brain abnormalities in our Tourette sample were not available, our findings on the significantly higher rates of minor physical anomalies couldn’t be matched with localized neuroanatomical abnormalities of the patients brain. Although there is a general consensus of a cortico-striatal-thalamo-cortical circuit abnormality, the pathophysiological locations are speculative26. Many investigators have focused on the striatal component5,32, however evidence is accumulating also to support a cortical dysfunction in Tourette syndrome33,34. As a next step of research a clinical comparison of Tourette patients with a high minor physical anomaly counts to patients with low counts should be evaluated in the terms of neuroanatomical findings, obstetrical lesions, familial neuropsychiatric disorders, level of IQ, learning disability and treatment response. We consider our data as important, either as a first step toward a possible exploration of a specific minor physical anomaly profile of Tourette patients or as indirect data supporting the neurodevelopmental hypothesis10,26,35 concerning the aetiology of combined vocal and multiple tic disorder.
We report on no conflict of interest.
References
1. Méhes K. Minor malformations in the neonate: Utility in screening infants at risk of hidden major defects. Prog in Clin and Biol Res 1985; 163: 45-49. [ Links ]
2. Trixler M, Tényi T, Csábi Gy, Szabó R. Minor physical anomalies in schizophrenia and bipolar affective disorder. Schizophr Res 2001; 52: 195-201. [ Links ]
3. Mink JW. Basal ganglia dysfunction in Tourette’s syndrome: A new hypothesis. Pediatr Neurol 2001; 25: 190-198. [ Links ]
4. Peterson BS, Riddle MA, Cohen DJ, Katz LD, Smith JC, Hardin MT. Reduced basal ganglia volumes in Tourette’s syndrome using three-dimensional reconstruction techniques from magnetic resonance images. Neurol 1993; 43: 941-949. [ Links ]
5. Singer HS, Reiss AL, Brown JE, Aylward EH, Shih B, Chee E. Volumetric MRI changes in basal ganglia of children with Tourette’s syndrome. Neurol 1993; 43: 950-956. [ Links ]
6. Gerard E, Peterson BS. Developmental processes and brain imaging studies in Tourette syndrome. J Psychosom Res 2003; 55: 13-22. [ Links ]
7. Hyde TM, Stacey MF, Coppola R, Handel SF, Rickler KC, Weinberger DR. Cerebral morphometric abnormalities in Tourette’s syndrome: a quantitative MRI study of monozygotic twins. Neurol 1995; 45: 1176-1182. [ Links ]
8. Peterson BS, Leckman JF, Duncan JS, Ketzles R, Riddle MA, Hardin MT. Corpus callosum morphology from magnetic resonance images in Tourette’s syndrome. Psych Res Neuroimag 1994; 55: 85-99. [ Links ]
9. Keshevan MS, Murray RM, editors. Neurodevelopment and Adult Psychopathology. Cambridge, New York, Melbourne: Cambridge University Press; 1997. [ Links ]
10. Ryan SG. Genetic susceptibility to neurodevelopmental disorders. J Child Neurol 1999; 14: 187-195. [ Links ]
11. Pinsky L. Informative morphogenetic variants. Minor congenital anomalies revisited. Iss Rev Teratology 1985; 3: 135-170. [ Links ]
12. Aase JM. Diagnostic Dysmorphology. New York, London: Plenum Medical Book Company; 1990. [ Links ]
13. Méhes K. Informative morphogenetic variants in the newborn. (English Edition by the Hungarian Academy of Sciences). Budapest: Akadémiai Kiadó; 1988. [ Links ]
14. Opitz JM. Editorial comment: Heterogeneity and minor anomalies. Amer J Med Gen 2000; 91: 254-255. [ Links ]
15. Gualtieri CT, Adams A, Shen CD, Loiselle D. Minor physical anomalies in alcoholic and schizophrenic adults and hyperactive and autistic children. Amer J Psychiat 1982; 139: 640-643. [ Links ]
16. Lohr JB, Flynn K. Minor physical anomalies in schizophrenia and mood disorders. Schizophr Bull 1993; 19: 551-556. [ Links ]
17. Lane A, Kinsella A , Murphy P, Byrne M, Keenan J, Colgan K, et al. The antropometric assessment of dysmorphic features in schizophrenia as an index of its developmental origins. Psychol Med 1997; 27: 1155-1164. [ Links ]
18. Trixler M, Tényi T, Csábi Gy, Szabó G, Méhes K. Informative morphogenetic variants in patients with schizophrenia and alcohol-dependent patients: Beyond the Waldrop Scale. Amer J Psychiat 1997; 154: 691-693. [ Links ]
19. Trixler M, Tényi T. Problems with the Waldrop scale. Amer J Psychiat 2000; 157: 486. [ Links ]
20. Waldrop MF, Goering JD. Hyperactivity and minor physical anomalies in elementary school children. Amer J Orthopsychiat 1971; 41: 602-607. [ Links ]
21. Feingold M, Bossert WH. Normal values for selected physical parameters: An aid to syndrome delineation. Birth defects 1974; 10/13: 1-16. [ Links ]
22. Merlob P. Mild errors of morphogenesis one of the most controversial subjects in dysmorphology. Iss Rev Teratology 1994; 7: 57-102. [ Links ]
23. Spranger J, Berirschke K, Hall JG, Lenz W, Lowry RB, Opitz JM, et al. Errors of morphogenesis: Concepts and terms. J Pediatr 1982; 100: 160-165. [ Links ]
24. Opitz JM. Invited editorial comment: Study of minor anomalies in childhood malignancy. Eur J Pediatr 1985; 144: 252-254. [ Links ]
25. Tényi T, Trixler M, Csábi Gy, Jeges S. Minor physical anomalies in non-familial unipolar recurrent major depression. J Affect Disord 2004; 79: 259-262. [ Links ]
26. Singer HS.Tourette’s syndrome: from behaviour to biology. Lancet Neurol 2005; 4: 149-159. [ Links ]
27. Diagnostic and Statistical Manual of Mental Disorders, Revised Fourth Edition.Washington DC: American Psychiatric Association; 1994. [ Links ]
28. Schmidt H. Supernumerary nipples: prevalence, size, sex and side predilection - a prospective clinical study. Eur J Pediatr 1998; 157: 821-823. [ Links ]
29. Brown J, Schwartz RA.Supernumary nipples: an overview. Cutis 2003; 71: 344-346. [ Links ]
30. Smith DW, Gong BT. Scalp hair pattering: Its origin and significance relative to early brain and upper facial development. Teratol 1974; 9: 17-34. [ Links ]
31. Frias JL, Carey JC. Mild errors of morphogenesis. Adv in Pediatr 1996; 43: 27-75. [ Links ]
32. Peterson BS, Thomas P, Kane MJ, Scahill L, Zhang H, Bronen R, et al. Basal ganglia volumes in patients with Gilles de la Tourette syndrome. Arch Gen Psychiatry 2003; 60: 415-424. [ Links ]
33. Fredericksen KA, Cutting LE, Kates WR, Mostofsky SH, Singer HS, Cooper KL, et al. Disproportionate increases of white matter in right frontal lobe in Tourette syndrome. Neurology 2002 8; 58: 85-89. [ Links ]
34. Kates WR, Frederikse M, Mostofsky SH, Folley BS, Cooper K, Mazur-Hopkins P, Kofman O, Singer HS, Denckla MB, Pearlson GD, Kaufmann WE. MRI parcellation of the frontal lobe in boys with attention deficit hyperactivity disorder or Tourette syndrome. Psychiatry Res 2002; 30; 116: 63-81. [ Links ]
35. Gilbert D. Treatment of children and adolescents with tics and Tourette syndrome. J Child Neurol 2006; 21: 690-700. [ Links ]
![]() Correspondence:
Correspondence:
Tamás Tényi
7623 Pécs
Rét u.2. Hungary
Tel/Fax : 36-72-535-951
e-mail: tamas.tenyi@aok.pte.hu
Received: 14 February 2008
Revised: 7 July 2008
Accepted: 11 July 2008