My SciELO
Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Española de Enfermedades Digestivas
Print version ISSN 1130-0108
Rev. esp. enferm. dig. vol.110 n.3 Madrid Mar. 2018
https://dx.doi.org/10.17235/reed.2017.4981/2017
CASE REPORTS
Nonalcoholic steatohepatitis and hepatic adenomatosis: casual or causal relationship?
1Servicio de Aparato Digestivo. Hospital Universitario "12 de Octubre". Madrid, España
2Departamentos de Anatomía Patológica. Hospital Universitario "12 de Octubre". Madrid, España
3Servicio de Radiología. Hospital Universitario "12 de Octubre". Madrid, España
INTRODUCTION
Hepatic adenomatosis (HA) is a benign and uncommon disease defined as the presence of at least ten adenomas in a healthy liver 1. Although the cause is still unknown, HA has been related to hormonal treatments, storage diseases, vascular anomalies and conditions associated with a familial predisposition such as inherited errors of metabolism or maturity onset diabetes of the young (MODY). In this regard, an association between MODY and HA has been found in families with a germ line mutation of the gene that codes for hepatocyte nuclear factor 1-alpha (HNF-1α) 2. The coexistence of HA and nonalcoholic steatohepatitis (NASH) has recently been reported in two patients suffering from metabolic syndrome, suggesting that both conditions share a pathophysiological and etiological pathway 3) (4.
We report the case of a young woman with fructosemia and hepatic steatosis, with a genetic mutation in HNF-1α and no other metabolic risk factors. Multiple hepatic adenomas associated with steatohepatitis lesions were found during clinical follow-up.
CASE REPORT
A 32-year-old female was referred to our specialized medical consultation for patients with nonalcoholic fatty liver disease (NAFLD) in 2000. She had been previously diagnosed with fructosemia or hereditary fructose intolerance (HFI) (fructose 1-phosphate aldolase deficiency) which was well controlled with a fructose-free diet since childhood. She had also been diagnosed with massive macrovacuolar hepatic steatosis at the age of four (percutaneous liver biopsy in 1988). The patient reported no alcohol or medication intake, including oral contraceptive pills. She did not have any other metabolic risk factors. Liver enzymes were within the normal range and other causes of hepatic disease had been excluded.
In 2004, 16 years after the initial diagnosis, a second liver biopsy identified steatohepatitis lesions. The steatosis grade was similar but new lesions were identified, consisting of hydropic degeneration and inflammatory infiltrate without sinusoidal collagen deposition or fibrosis. During follow-up, the patient remained asymptomatic and the blood tests were within the normal range. In addition, successive ultrasound scans identified moderate steatosis, without any indirect signs of fibrosis (FibroScan and serologic markers).
In 2010, the patient complained of non-specific abdominal discomfort and an increased number of bowel movements; no specific cause was found after a general medical examination and tests. Subsequently, an abdominal magnetic resonance imaging (MRI) scan was performed and no liver lesions were identified other than fatty infiltration.
In 2012, after an uneventful pregnancy and delivery, an abdominal ultrasound scan identified multiple hepatic lesions suggestive of adenoma vs focal nodular hyperplasia. Another abdominal MRI confirmed the presence of at least 15 nodules with an adenoma-like morphology. One lesion had a subcapsular location and measured 17 mm. In addition, three other lesions were described; the largest one measured 34 mm and was suggestive of a focal nodular hyperplasia rather than an adenoma (Fig. 1). Due to the suspicion of a HA, an ultrasound scan guided microbiopsy was performed in 2013. Two cytological distinct zones were identified in the sample, one with steatohepatitis changes (similar to the ones described in the previous percutaneous biopsies) and another with a benign hepatocellular proliferation. This lesion was suggestive of an inflammatory adenoma according to the immunohistochemical study (Fig. 2 and Fig. 3). These lesions have remained stable in successive abdominal MRI and ultrasound scans.
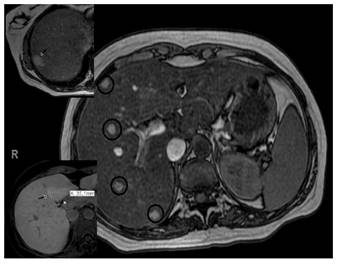
Fig. 1 Magnetic resonance imaging: hepatomegaly with severe steatosis and multiple nodules with hyper-uptake in the arterial phase and hypointense in the hepatocellular phase. These findings are suggestive of adenomas. Image details: subcapsular adenoma measuring 17 mm (top left picture); lesion on segment IV measuring 35 mm, highly suggestive of focal nodular hyperplasia (bottom left picture).
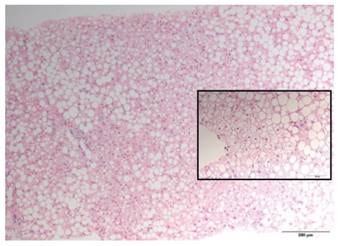
Fig. 2 Non-tumoral liver. Massive macrovacuolar steatosis (H&E stain; 200x). Image detail: hepatocellular ballooning. These findings are suggestive of steatohepatitis (H&E stain; 400x).

Fig. 3 A. Transition zone between liver parenchyma with steatohepatitis (left) and adenoma (right). Note that the adenoma zone shows no steatosis (H&E stain; 20 x). B. Immunohistochemical study. SAA staining: SAA-positive hepatocellular neoplasm (top right picture) (20x). ß-catenin staining: negative for nuclear staining (bottom right picture) (200x).
The HNF-1α germline mutation was analyzed and the patient was a heterozygotic carrier. Even though the levels of glucose, insulin and glycated hemoglobin remained within the normal range during follow-up, a 75 gram oral glucose tolerance test had abnormal results in the most recent visit.
DISCUSSION
NAFLD is nowadays considered to be the most common cause of chronic liver disease in developed countries 5. This anatomic-clinical entity is characterized by the presence of liver lesions similar to those caused by alcohol but appearing in the absence of alcohol exposure. The term NAFLD describes a variety of lesions of varying severity, from simple steatosis and NASH with progressive grades of fibrosis to cirrhosis 6.
Although NAFLD is considered to be a hepatic manifestation of metabolic syndrome and insulin resistance (primary NAFLD) in the majority of cases, some cases are considered to be secondary to NAFLD. These include conditions related to medication intake or diseases such as hypothyroidism, polycystic ovary syndrome, sleep apnea-hypopnea syndrome, inflammatory bowel disease, hyperuricemia and gout 6) (7.
With regard to pediatric NAFLD, obesity and other diseases have been traditionally considered as possible etiologic factors. These include some hereditary metabolism defects (both carbohydrate and lipid) such as galactosemia, glycogenosis, abetalipoproteinemia and fructosemia (diagnosed in this case).
Some cases of HA associated with hepatic fat deposits have been described previously. However, only two patients with NASH lesions and HA have been reported to date, both middle aged women suffering from metabolic syndrome. The authors that described these cases also speculated about the possibility of both entities sharing a causal mechanism 3) (4.
HA is a benign disease characterized as the presence of multiple adenomas in a normal liver. At least ten adenomas are required to establish a diagnosis 8. This is an uncommon condition, with less than 100 cases reported to date 9. The etiopathogenic mechanism of HA is still unclear. It has been associated with the oral contraceptive pill, anabolic steroid use, vascular anomalies such as focal nodular hyperplasia, certain storage diseases, inherited metabolism defects and diabetes mellitus (DM) 8) (9. In this regard, a germ line mutation of the HNF-1α gene has been described in families with HA and MODY 2.
Our patient suffered from hereditary fructose intolerance and has followed a strict fructose-free diet since childhood. Therefore, this condition should not influence the progression from steatosis to steatohepatitis. In addition, there were no metabolic risk factors, nor use of any of the NASH related medications. The heterozygotic status of the HNF-1α germ line mutation could have promoted the development of HA and the onset of glucose intolerance. In this regard, bearing in mind the pathogenic association of a high fructose occidental diet with the NAFLD, it is possible that the hereditary fructose intolerance in this case could have been a NASH protection factor. Indeed, the substitution of high-fructose foods with glucose-containing foods could have favored the development of glucose intolerance. Even though HA has been associated with hereditary metabolism defects such as galactosemia, glycogenosis and tyrosinemia, there is no data with regard to the possible association with fructosemia that was present in this case 9.
Even though the progression of untreated HA is not well understood, it is known that adenomas larger than 50 mm have the greatest risk of complications, especially bleeding and malignant degeneration 10. It is possible to define the various categories of adenoma and establish the risk of progression to hepatocarcinoma via histological and immunohistochemical studies. Therefore, nuclear expression of a mutant form of the ß-catenin gene in adenomatous tissue is linked to malignancy and, therefore, is an indication for surgical resection 1) (10. In this case, the histological study of the microbiopsy of the largest lesion confirmed that it was an adenoma. It was classified as an inflammatory hepatocellular adenoma (amyloid A-positive) according to the immunohistochemical marker analysis with a minimal risk of becoming malignant (lack of activated nuclear -catenin).
Following the current recommendations, our patient has been periodically monitored via imaging tests. Therefore, it is possible to plan a surgical resection or a transarterial embolization if any complications develop or if there is growth of more than 10 mm of any of the current lesions 9) (10.
The avoidance of pregnancy is only recommended in cases of adenomas larger than 50 mm. However, the absence of hepatic lesions identified by imaging prior to becoming pregnant and their development one year later support the decision to avoid further pregnancies, in case the hormonal changes influence the evolution of HA. Since there is only limited information available regarding the association of HA and NASH, we can only follow the recommendations from the clinical practice guidelines for the management of NAFLD 5. Our patient required closer supervision of her glucose metabolism due to the risk of developing a MODY diabetes mellitus.
In summary, we present the case of a young female patient suffering from fructosemia and hepatic steatosis who was also diagnosed with HA and NASH years after the initial diagnosis. Although a HNF-1α germ line mutation and hormonal changes related to pregnancy could be HA promoting factors, there is no evidence of an association between any of these conditions and the development of NASH. In accordance with other authors, we consider that reporting patients with HA and NASH will help to clarify whether these conditions share a causal mechanism or if their association is the result of a mere coincidence.
BIBLIOGRAFÍA
1. Donato M, Jahromi AH, Andrade AI, et al. Hepatic adenomatosis: A rare but important liver disease with severe clinical implications. Int Surg 2015;100:903-7. DOI: 10.9738/INTSURG-D-14-00161.1 [ Links ]
2. Reznik Y, Dao T, Coutant R, et al. Hepatocytonuclear factor-1 gene inactivation: Consegregation between liver adenomatosis and diabetes phenotypes in two maturity-onset diabetes of the young (MODY) 3 families. J Clin Endocrinol Metabol 2004;83:1476-80. DOI: 10.1210/jc.2003-031552 [ Links ]
3. Brunt EM, Wolverson MK, Di Bisceglie AM. Benign hepatocellular tumors (adenomatosis) in nonalcoholic steatohepatitis: A case report. Sem Liver Dis 2005;25:230-6. DOI: 10.1055/s-2005-871202 [ Links ]
4. Nascimbeni F, Ballestri S. Inflammatory hepatocellular adenomatosis, metabolic syndrome, polycystic ovary syndrome and non-alcoholic steatohepatitis: Chance tetrad or association by necessity? Dig Liver Dis 2014;46:288-91. [ Links ]
5. EASL-EASD-EASO Clinical practice guidelines for the management of non-alcoholic fatty liver disease. J Hepatol 2016;64:1388-402. DOI: 10.1016/j.jhep.2015.11.004 [ Links ]
6. Solís Herruzo JA, García Ruiz I, Pérez Carreras M, et al. Non-alcoholic fatty liver disease. From insulin resistance to mitochondrial dysfunction. Rev Esp Enferm Dig 2006;98:844-74. DOI: 10.4321/S1130-01082006001100006 [ Links ]
7. Martín-Domínguez V, González-Casas R, Mendoza-Jiménez Ridruejo J, et al. Pathogenesis, diagnosis and treatment of non-alcoholic fatty liver disease. Rev Esp Enferm Dig 2013;105:409-20. DOI: 10.4321/S1130-01082013000700006 [ Links ]
8. Flejou JF, Barje J, Menu Y, et al. Liver adenomatosis: An entity distinct from liver adenoma? Gastroenterology 1985;89:891132-8. [ Links ]
9. Lucas E, Pareja E, Carvajal N, et al. Adeomatosis hepática: una enfermedad de tratamiento controvertido. Cir Esp 2014;92:284-96. DOI: 10.1016/j.ciresp.2013.09.006 [ Links ]
10. Fernández-Vega I, Santos-Juanes J, García-Pravia C, et al. Hepatic adenomatosis: A rare cause of liver transplant. Rev Esp Enferm Dig 2014;106: 494-6. [ Links ]
Received: April 09, 2017; Accepted: October 22, 2017











 text in
text in 

