My SciELO
Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Española de Enfermedades Digestivas
Print version ISSN 1130-0108
Rev. esp. enferm. dig. vol.110 n.4 Madrid Apr. 2018
https://dx.doi.org/10.17235/reed.2018.5245/2017
ORIGINAL PAPERS
Viability of single balloon enteroscopy performed under endoscopist-directed sedation
1Servicio de Aparato Digestivo. Hospital Universitario Lucus Augusti. Lugo, España
INTRODUCTION
Nowadays, sedation is an inseparable part of any digestive endoscopic procedure, as there is general agreement that it must be offered to every patient whilst explaining the risks, advantages, disadvantages and alternatives 1. There is a lot of controversy with regard to who must be responsible for the sedation and monitoring of the patient during digestive endoscopy explorations. The international endoscopy scientific societies and also the Spanish Society of Digestive Endoscopy (SEED) have developed clinical practice guidelines that clearly specify different levels of sedation, drugs that can be used and in which situations, as well as the limits of sedation directed by non-anesthetists. It is accepted that the use of superficial sedation is sufficient for basic endoscopic procedures and that the endoscopist and/or the nursing staff can be responsible for sedation. However, deep sedation preferably with propofol is recommended for advanced endoscopy procedures, in contrast to traditional sedation based on benzodiazepines and opioids (1-9). In Spain, there has been a lot of controversy for more than a decade about who should be in charge of sedation, particularly when propofol is used. The main characters involved in the issue have still not reached an agreement. As a result, there is a wide variability in sedation protocols among different hospitals 10,11,12,13.
Enteroscopy, both double-balloon and single-balloon, is increasingly used for the diagnosis and treatment of certain diseases of the small intestine. It is an advanced endoscopic technique due to its prolonged duration and also potentially painful. Overtubes are necessary, and therefore instrumental risk is increased, the procedure requires deep sedation and is frequently accompanied by therapeutic maneuvers of varying complexity. In Spain, it is very common that this exploration is carried out under deep sedation controlled by an anesthetist and even under general anesthesia with orotracheal intubation.
The aim of the study was to explore the viability of performing single-balloon enteroscopy under endoscopist-directed sedation, evaluating the possibility to complete the scheduled procedure and also its efficacy.
MATERIAL AND METHOD
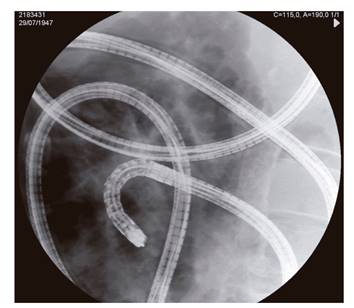
This was a prospective, observational study and every single-balloon enteroscopy performed in our hospital from January 2014 to July 2017 was recorded. Every procedure was performed in an outpatient basis using an Olympus(r) enteroscope with a single balloon overtube. The clinical staff that participated in the procedure included an endoscopist, a scrub nurse and a nurse in charge of monitoring and sedative administration following the endoscopist's instructions. Arterial oxygen saturation, cardiac rate, blood pressure, electrocardiogram and respiratory rate were monitored. CO2 was used for bowel insufflation. Continuous supplemental oxygen was administered through a nasal cannula with a flow rate of four liters per minute. Every enteroscopy was performed under fluoroscopic control and with the patient in the left lateral decubitus (Fig. 1). The exclusion criteria included age under 18, pregnancy, endoscopic exploration contraindications and a lack of consent.
The sedative drugs used that were selected by the endoscopist included propofol alone with an infusion pump or in combination with midazolam and/or fentanyl, or a fentanyl and midazolam combination. The choice was made according to the examiners' preferences, patients' medical history (anxiety, alcohol or drugs consumption, etc.) and evolution of the procedure. This was mainly related with the need to administer repeat doses of the sedative drug due to an insufficient level of sedation or the onset of pain.
Patient comorbidities, medical history, allergies, drugs or alcohol consumption and previous episodes of anesthesia or sedation complications were recorded before starting sedation. All patients went to the Endoscopy Unit accompanied by an adult and signed two consent forms, one for the enteroscopy itself and the other for sedation. A brief physical examination was performed before the procedure, paying special attention to neurologic and cardiorespiratory status. The patients were classified according to the American Association of Anesthesia (ASA) Physical Status Classification for anesthetic risk assessment.
The following parameters were recorded for the study: age, sex, weight, comorbidities, ASA class, indication for enteroscopy, drugs and the doses used, distance of insertion beyond the angle of Treitz or the ileocecal valve, length of the procedure, findings, therapeutic maneuvers and complications. Monitoring alarms for complications were set at the following levels: cardiac rate, 50-120 per minute; systolic blood pressure, 90-160 mmHg; respiratory rate, 10-30 per minute; and arterial oxygen saturation, < 90% (mild desaturation was between 80 and 90% and severe desaturation, < 80%).
After the procedure, patients were monitored in a recovery room until they were discharged, after having reached a score of 9 or 10 on the Aldrete scale. All patients had a check-up appointment within a period of no more than one month.
Statistical analysis of the data was performed using the SPSS version 19 software (IBM; SPSS Inc., Chicago IL, USA). The Student's t-test was used to compare continuous variables and the Chi-squared test or Fischer's exact test were used to compare categorical variables. All statistic tests were two-tailed and p < 0.05 was considered as significant.
RESULTS
Forty-four enteroscopies were performed on 39 patients, 24 males and 15 females. The median age was 74 years (18-89) and the median weight was 70 kg (51-105). Every patient fulfilled the inclusion criteria and no patients were excluded. The anesthetic risk was as follows: ASA1, 12 patients; ASA II, 23 patients; and ASA III, nine patients. Comorbidities were as follows: none, 14 cases; heart disease, 18; chronic kidney failure, two; cirrhosis of the liver, two; heart disease and COPD, one; heart disease and chronic kidney failure, one; diabetes, one; and COPD, one.
Indications for enteroscopy were as follows: gastrointestinal bleeding of an obscure origin, 26; anemia, five; abdominal pain, four; chronic diarrhea two; constitutional syndrome, two; ileitis, one; Peutz Jehgers syndrome, two; cancer suspicion, one; and postpolypectomy control, one.
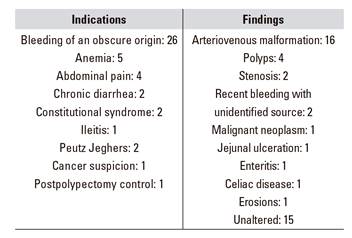
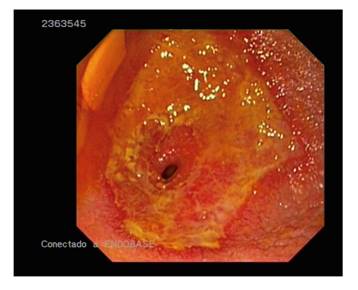
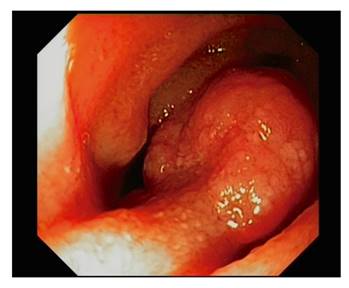
The enteroscopy findings were as follows: arteriovenous malformation, 16; polyps, four; stenosis, two; recent bleeding with unidentified source, two; malignant neoplasm, one; jejunal ulceration, one; enteritis, one; celiac disease, one; erosion, one; and no alterations, 15 (Table 1 and Fig. 2 and Fig. 3).
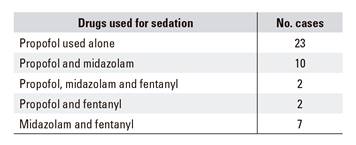
Thirty-six enteroscopies were anterograde and eight were retrograde. The median of the explored tract length was100 cm (30-200) and the median length of the procedure was 52 minutes (20-120). Therapeutic maneuvers were performed in 21 cases, argon electrocoagulation in 15 cases, balloon dilation in two cases and polypectomy in four cases. The drugs and doses used for sedation are shown in table 2:
Propofol was used alone in 23 cases and the median dose was 330 mg (80-705).
Propofol and midazolam was used in ten cases and the median dose was 446 mg (196-600) of propofol and 2.5 mg (1-5) of midazolam.
Propofol, midazolam and fentanyl were used in two cases and the median dose of propofol was 152 mg (20-285); of midazolam, 6.5 mg (5.5-7.5); and of fentanyl, 0.05 mg.
Propofol and fentanyl were used in two cases. The median dose of propofol was 546 mg (191-901) and of fentanyl, 0.05 mg.
Midazolam and fentanyl were used in seven cases and the median dose of midazolam was 5 mg (3.75-7.5) and of fentanyl, 0.10 mg (0.05-0.10).
Except for the seven cases in which midazolam and fentanyl were used from the beginning, sedation was started with propofol, then midazolam and/or fentanyl was added later when necessary. This was decided according to endoscopists' criteria of a sufficient level of sedation, midazolam was used instead of increasing the propofol dose and fentanyl was administered in the case of pain.
Ten complications were recorded and these included the following: mild arterial desaturation in two cases, hypotension in three cases, bradycardia in two cases and hypertension in three cases. All complications were easily resolved spontaneously or with simple maneuvers such as the jaw-thrust technique, an increase in oxygen flow rate, saline infusion or administration of a low atropine dose, and no extraordinary measures were required.
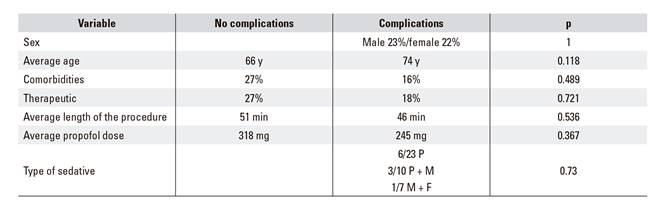
The average propofol dose when administered alone (338.57 mg) with the average dose used when associated with midazolam (410.30 mg) was compared and the difference was not statistically significant (p = 0.269). The relationship between sex, age, comorbidities, therapeutic maneuvers, length of the procedure, propofol dose and the type of sedative used and the occurrence of complications was also analyzed but it was not significant (Table 3). No procedures were cancelled or ended ahead of schedule. No patients required hospitalization after the procedure and no complications were reported within 30 days of the procedure.
DISCUSSION
This study aimed to assess if deep enteroscopy can be performed safely and effectively when sedation is administered by the endoscopy team, without an anesthetist. We prospectively recorded the results obtained from the 44 consecutive explorations referred to the Endoscopy Unit due to common indications for this procedure. The monitoring and sedation protocol was the same as that used for advanced endoscopy in our unit. A trained nurse was exclusively assigned to the monitoring and administration of sedation and analgesia under the guidance of the endoscopist. There is very little published information about sedation in enteroscopy. Although there are some studies of endoscopist-directed sedation with benzodiazepines and opioids, most of the publications affirm that anesthetists are responsible and that deep sedation or general anesthesia are used 16.
In terms of efficacy, our data are similar to those described in other series, with all explorations completed, a diagnosis success rate of 65.9% and therapeutic maneuvers performed in 47.75% of patients. The diagnosis success and therapeutic maneuvers rates were 41-65% and 7-50%, respectively, in a recent study 17. With regard to the safety of the endoscopic technique, this study had satisfactory results and there were no related complications. This is in accordance with published studies which report very low enteroscopy complication rates, in the order of 1 to 1.6% 18,19,20. Finally, it is worth noting that no complications were reported that arose from sedation; this was one of the objectives of this study. Minor complications were reported that were resolved with simple maneuvers in 22.7% of cases. It was not necessary to interrupt the procedure, intubate the patient or perform resuscitation maneuvers in any case. This is particularly important considering that 68% of the patients had comorbidities and that the median age was high at 74 years. Once again, these figures are similar to those of published studies which reported minor complications derived from sedation in 14-33% of the cases 21,22,23.
The quality parameters that must be considered when assessing who is responsible for sedation during digestive endoscopic procedures include efficacy, safety and efficiency. At present, there is a large amount of scientific evidence that demonstrates that properly trained non-anesthetist professionals with suitable materials obtain high levels of these parameters, not only in basic endoscopy but also in advanced endoscopy 24,25,26,27,28,29. In the case of deep enteroscopy, only a few studies have been published that specifically refer to sedation. Different patterns of sedation were assessed, including the use of benzodiazepines and opioids, propofol associated or not with pentazocine, anesthesia with or without orotracheal intubation that was administered by endoscopists and trained nurses in some cases and by anesthetists in other cases. The results regarding efficacy and safety are satisfactory, with little variation among the different regimes and a tendency for more complications with a longer procedure length or when anesthesia is employed 22. Judah JR et al. compared the results of nurse-administered and anesthetist-administered sedation during spiral enteroscopy in a series of 91 patients. This study found that there were no significant differences between both groups except for a shorter time of the procedure when sedation was administered by nurses (39 minutes vs 46) and a higher rate of findings when administered by anesthetists (74.1% vs 50%) 30.
With regard to efficiency, and even though it was not the objective of this study, it is clear that healthcare cost is substantially lower if sedation is performed by non-anesthetists. This has been confirmed by other studies 31,32. In this regard, it must be pointed out that in this study every procedure was performed on an outpatient basis, without hospitalization or special unit occupation.
Our series appears to endorse that duly trained and well equipped non-anesthetist staff can be responsible for sedation during enteroscopy with an overtube, at least in low anesthetic risk patients. Nevertheless, we must acknowledge that our study was carried out in only one center and included a small number of procedures. For these reasons, it would be interesting to perform research studies with more hospitals and including a higher number of cases, so that solid conclusions can be drawn.
ACKNOWLEDGMENTS
The authors thank Pilar Lancho for the translation of the manuscript into English.
REFERENCES
1. Igea F, Casellas JA, González-Huix F, et al. Sedation for gastrointestinal endoscopy. Endoscopy 2014;46:720-31. DOI: 10.1055/s-0034-1377561 [ Links ]
2. Anesthesiology. Practice guidelines for sedation and analgesia by non-anesthesiologists. American Society of Anestesthesiologists Task Force on Sedation and Analgesia by Non Anesthesiologists. Anesthesiology 2002;96:1004-17. DOI: 10.1097/00000542-200204000-00031 [ Links ]
3. Gastrointestinal Endoscopy (GIE). Guidelines for the use of deep sedation and anesthesia for GI endoscopy. American Society for Gastrointestinal Endoscopy. Gastrointest Endosc 2002;56:613-16. [ Links ]
4. ASGE Training Committee. Training in patient monitoring and sedation and analgesia. Communication from the ASGE Training Committee. Gastrointest Endosc 2007;66:7-10. [ Links ]
5. ASGE Guideline. Sedation and anesthesia in GI endoscopy. ASGE Guideline. Gastrointest Endosc 2008;68:815-26. [ Links ]
6. Vargo JJ, Cohen LB, Rex DK, et al. Position statement: nonanesthesiologist administration of propofol for GI endoscopy. Gastrointest Endosc 2009;70:1053-9. DOI: 10.1016/j.gie.2009.07.020 [ Links ]
7. Jain R, Ikenberry SO, Anderson MA, et al. ASGE Standards of Practice Committee. Minimum staffing requirements for the performance of GI endoscopy. Gastrointest Endosc 2010;72:469-70. DOI: 10.1016/j.gie.2010.02.017 [ Links ]
8. Vargo JJ, DeLegge MH, Feld AD, et al. Multisociety sedation curriculum for gastrointestinal endoscopy. Gastrointest Endosc 2012;76:1-25. DOI: 10.1016/j.gie.2012.03.001 [ Links ]
9. European Society of Gastrointestinal Endoscopy (ESGE). European Curriculum for Sedation Training in Gastrointestinal Endoscopy: Position Statement of the European Society of Gastrointestinal Endoscopy (ESGE) and European Society of Gastroenterology and Endoscopy Nurses and Associates (ESGENA). Endoscopy 2013;45:495-503. [ Links ]
10. Campo R, Brullet E, Junquera F, et al. Sedación en la endoscopia digestiva. Resultados de una encuesta hospitalaria en Cataluña. Gastroenterol Hepatol 2004;27(9):203-7. DOI: 10.1016/S0210-5705(03)70516-0 [ Links ]
11. Cubiella Fernández J, Lancho Seco A, Echarri Piudo A, et al. Sedación en las unidades de endoscopia de Galicia. Resultados de la encuesta de la Sociedad Gallega de Patología Digestiva. Rev Esp Enf Dig 2005;97(1): [ Links ]
12. Baudet JS, Borque P, Borja E, et al. Use of sedation in gastrointestinal endoscopy: a nationwide survey in Spain. Eur J Gastroenterol Hepatol 2009;21:882-8. DOI: 10.1097/MEG.0b013e328314b7ca [ Links ]
13. Lucendo AJ, González-Huix F, Tenias JM, et al. Spanish Society of Digestive Diseases, Spanish Society of Digestive Endoscopy, and Spanish Association of Gastroenterology. Gastrointestinal endoscopy sedation and monitoring practices in Spain: a nationwide survey in the year 2014. Endoscopy 2015;47(4):383-90. DOI: 10.1055/s-0034-1391672 [ Links ]
14. Pérez-Cuadrado E, Más P, Hallal H, et al. Double-balloon enteroscopy: a descriptive study of 50 explorations. Rev Esp Enf Dig 2006;98(2):73-81. DOI: 10.4321/S1130-01082006000200002 [ Links ]
15. Pérez-Cuadrado-Robles E, Esteban-Delgado P, Martínez-Andrés B, et al. Diagnosis agreement between capsule endoscopy and double-balloon enteroscopy in obscure gastrointestinal bleeding at a referral center. Rev Esp Enf Dig 2015;107(8):495-500. [ Links ]
16. Riccioni ME, Urgesi R, Cianci R, et al. Current status of device-assisted enteroscopy: technical matters, indication, limits and complications. World J Gastrointest Endosc 2012;4(10):453-61. DOI: 10.4253/wjge.v4.i10.453 [ Links ]
17. ASGE Technology Committee. Technology status evaluation report. Enteroscopy. Gastrointest Endosc 2015;82(6):975-990. [ Links ]
18. ASGE Technology Committee. Enteroscopes. Gastrointest Endosc 2007; 66:876-80. [ Links ]
19. Mensink PB, Haringsma J, Kucharzik T, et al. Complications of double balloon enteroscopy: a multicentre survey. Endoscopy 2007;39:613-5. DOI: 10.1055/s-2007-966444 [ Links ]
20. Gerson LB, Tokar J, Chiorean M, et al. Complications associated with double balloon enteroscopy at nine US centers. Clin Gastroenterol Hepatol 2009;7:1177-82. DOI: 10.1016/j.cgh.2009.07.005 [ Links ]
21. Treeprasertsuk S, Rerknimitr R, Angsuwatcharakon P, et al. The safety of propofol infusion compared to midazolam and meperidine intravenous bolus for patients undergoing double balloon enteroscopy. J Med Assoc Thai 2014;97(5):483-9. [ Links ]
22. Sethi S, Thaker AM, Cohen J, et al. Monitored anesthesia care without endotracheal intubation is safe and efficacious for single-balloon enteroscopy. Dis Dis Sci 2014;59(9):2184-90. DOI: 10.1007/s10620-014-3118-2 [ Links ]
23. AK Journals. General anesthesia during double balloon enteroscopy. The Hungarian experiences. Orv Hetil 2010;151(48):1976-82. [ Links ]
24. Jung M, Hofmann C, Kiesslich R, et al. Improved sedation in diagnostic and therapeutic ERCP: propofol is an alternative to midazolam. Endoscopy 2000;32:233-8. DOI: 10.1055/s-2000-96 [ Links ]
25. Wehrmann T, Kokapick S, Lembcke B, et al. Efficacy and safety of intravenous propofol sedation for routine ERCP: a prospective controlled study. Gastrointest Endosc 1999;49:677-83. DOI: 10.1016/S0016-5107(99)70281-6 [ Links ]
26. Vargo JJ, Zuccaro G, Dumot JA, et al. Gastroenterologist-administered propofol versus meperidine and midazolam for ERCP and EUS: a randomized, controlled trial with cost effectiveness analysis. Gastroenterology 2002;123:8-16. DOI: 10.1053/gast.2002.34232 [ Links ]
27. Goudra BG, Singh PM, Gouda G, et al. Safety of non-anesthesia provider-administered propofol (NAAP) sedation in advanced gastrointestinal endoscopic procedures: comparative meta-analysis of pooled results. Dig Dis Sci 2015;60(9):2612-27. DOI: 10.1007/s10620-015-3608-x [ Links ]
28. Wadhwa V, Issa D, Garg S, et al. Similar risk of cardiopulmonary adverse events between propofol and traditional anesthesia for gastrointestinal endoscopy: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2017;15(2):194-206. DOI: 10.1016/j.cgh.2016.07.013 [ Links ]
29. Pérez-Cuadrado-Robles E, González-Ramírez A, Lancho-Seco A, et al. Safety and risk factors for difficult endoscopist-directed ERCP sedation in daily practice: a hospital-based case-control study. Rev Esp Enf Dig 2016;108:240-5. DOI: 10.17235/reed.2016.4206/2016 [ Links ]
30. Judah JR, Collins D, Gaidos JK, et al. Prospective evaluation of gastroenterologist-guided, nurse-administered standard sedation for spiral deep small bowel enteroscopy. Dig Dis Sci 2010;55(9):2584-91. DOI: 10.1007/s10620-010-1335-x [ Links ]
31. Aisenberg J. Sedation for gastrointestinal endoscopy: new practices, new economics. Am J Gastroenterol 2005;996-1000. DOI: 10.1111/j.1572-0241.2005.50034.x [ Links ]
32. Hassan C, Rex DK, Cooper GS, et al. Endoscopist-directed propofol administration versus anesthesiologist assistance for colorectal cancer screening: a cost effectiveness analysis. Endoscopy 2012;44(5):456-64. DOI: 10.1055/s-0032-1308936 [ Links ]











 text in
text in