Introduction
Hepatitis C virus (HCV) represents one of the greatest public health problems, which affects over 180 million persons, that is to say 3% of the world population1. Between 50 and 80% of patients will develop chronic Hepatitis C, and approximately 20% of these cases will end up in cirrhosis within 25 to 40 years; currently, Hepatitis C is the main cause of death by liver condition and of liver transplant2. The most likely cause for getting infected in the 60s and 70s was the widespread use of transfusion of blood products in clinical practice. In the 80s and 90s, the boom in parenteral use of drugs triggered a major increase in the rates of other infectious diseases such as HIV. This leads to a noticeable increase in patients with advanced liver disease nowadays, which will lead to an increase in health-care expenses during the next two decades. Standard treatment consisted in the combination of Pegylated interferon and ribavirin (PegIFN+RBV), and it achieved cure rates close to 60% from all cases treated, measured by the Sustained Viral Response (SVR) rates obtained3. Obviously, there was room for improvement in this response rate, particularly in patients with Genotype 1, the most numerous population in our setting, because there was no response to treatment in >50% of patients. Among the factors associated with this low response rate, we can highlight those associated with the virus (high viral load and genotype), and with patients (CT/TT genotypes of the interleukin-28B gene, insulin resistance, obesity, co-infection with other virus, and advanced fibrosis)4-5. The launch of the first generation of new antivirals acting directly on the replication cycle of the virus C, called Direct Antiviral Agents (DAAs) represented a dramatic advance in terms of increase in cure rates. SVR rates of 66-79% were achieved in naïve patients. Outcomes were also hopeful in those patients with failure to PegIFN+RBV, with good results in patients with relapses: 75-84%, partial responders: 52-61%, and patients with no previous response (telaprevir only): 31%4-8. The launch of boceprevir and telaprevir represented a revolution in the pharmacological setting for this disease, and triple therapy with a protease inhibitor of the Hepatitis C virus, in combination with peg-interferon and ribavirin, became the treatment of choice for patients with Chronic Hepatitis C and Genotype 1.
However, currently these drugs have fallen into disuse due to their profile of adverse reactions, their high cost per SVR in patients with advanced fibrosis, and the launch of the so-called second generation DAAs, with dramatic response rates and an unbeatable profile of adverse reactions9. These second generation drugs allow a reduction in the duration of treatment, and a less complex monitoring than preceding drugs. On the contrary, the high cost and great variety of treatments involve a careful selection of patients to be treated, and the choice of the optimal treatment for each individual10.
The unquestionable current impact on hospitals, both in patient care and pharmacoeconomic terms, of the launch of second generation DAAs, forces us to achieve an optimization and consensus in the use of these drugs, and to focus on aspects so far not adequately valued, like health outcomes perceived by patients (PROs = Patient Reported Outcomes). This aspect is closely linked to an individualized high-quality pharmacotherapeutical follow-up, based on an innovative model of pharmaceutical care. This model combines the basic cornerstones, such as the reinforcement of treatment adherence and stratification based on overall pharmacotherapeutical complexity, with aspects associated with quality of life and satisfaction which have become important in recent years, and provide the experience of patients with their disease and its treatment.
There are studies measuring quality of life and satisfaction with the standard treatment of peg-interferon + Ribavirin ± first generation DAA11,12 , but there are few studies assessing the impact of treatment with second generation DAAs, regardless of their lower complexity and better tolerability, on health outcomes perceived, or more specifically, measuring to what extent patients are satisfied with new treatments.
The objective of this study is to determine the relationship between the complexity of treatment for Hepatitis C and patient satisfaction.
Method
An observational, analytical, prospective, single-centre study, including >18-year-old patients with diagnosed infection by HVC, who had received at least 4 weeks of treatment with DAA, and had attended the Pharmaceutical Care Unit of a General Hospital between October 2014 and February 2016 was conducted. Those patients included in clinical trials during the study period were excluded, as well as patients whose follow-up had been discontinued for any reason, or those who did not complete the relevant Informed Consent.
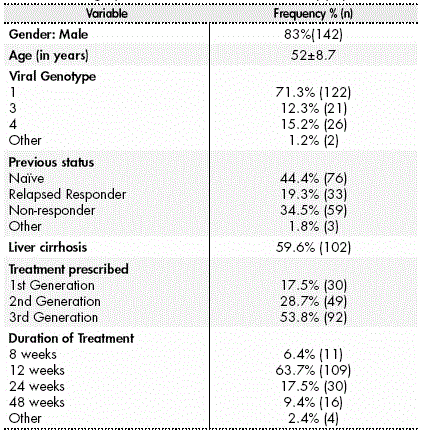
Demographical variables were collected (age, gender), as well as variables regarding Hepatitis C, such as viral genotype and the presence or not of liver cirrhosis, and the status before treatment initiation. Patients were classified into naïve, relapsing responders (RR) or non-responders (NR) to previous treatments.
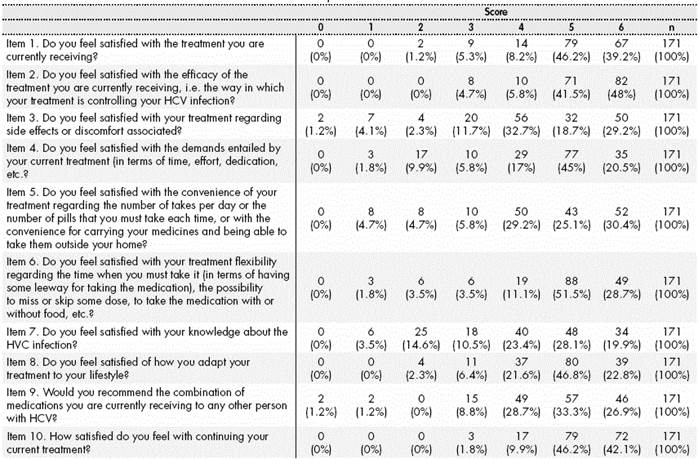
The primary endpoint of the study was the assessment of satisfaction with treatment for Hepatitis C, measured through the ESTAR questionnaire13 adapted to the patient population with Hepatitis C. This tool included 10 questions to be answered with a Likert scale, from 0 (Not at all satisfied) to 6 (Extremely Satisfied); therefore, overall satisfaction with treatment for Hepatitis C ranged between 0 and 60 scores. The questionnaire was structured into two dimensions: Clinical Satisfaction (items 1,2,3,9 and 10) and Satisfaction with Lifestyle (items 4,5,6,7,8). The first section addressed aspects about satisfaction with treatment: its efficacy, adverse reactions and requirements entailed, likely recommendation to other patients, and willingness to continue their current treatment. The section regarding lifestyle analyzed matters associated with treatment convenience and flexibility, awareness about the disease, and adaptation of treatment to their lifestyle. There was also a qualitative assessment of this satisfaction score: <50/ >=50 scores, low or high satisfaction, respectively.
At the same time, there was a reliability analysis of the questionnaire used to assess the quantitative satisfaction of patients with treatment for HCV, in a preliminary analysis with the first 30 patients of the study, and subsequently with the complete sample. To this aim, there was an estimation of Cronbach’s alpha coefficient, and the intraclass correlation coefficient was also measured.
Regarding pharmacotherapy, the data collected were: treatment selected for HVC, concomitant medication, and SVR.
The combination of drugs prescribed was obtained through the program for outpatient dispensing by the Pharmacy Unit (Dominion-Farmatools®), and was classified, for its better management, into three generations of treatment. First generation drugs included boceprevir and telaprevir, second generation included combinations with sofosbuvir and standard therapy or ribavirin only, and combinations with simeprevir or daclatasvir. Finally, third generation drugs were: sofosvubir/ledipasvir and ombitasvir/parataprevir/ritonavir with or without dasabuvir.
Concomitant treatment was collected from the XXI Prescription Application by the Andalusian Health System. The rest of variables were obtained by computer search in the Single Clinical Patient Record.
The complexity index of the complete pharmacotherapy was calculated through the MRCI computer application by the University of Colorado14. Additionally, there was an estimation of the scores for the specific complexity of the treatment against HCV and the complexity index of the concomitant medication prescribed. In order to detect an estimated 2-score difference in the complexity index of the specific medication against HCV between both groups of patients, according to the qualitative assessment of satisfaction (high/low) based on the ESTAR Questionnaire, a 4-score variability was considered common to both groups, a 5% alpha error, an 80% potency, and an expected 5% loss; this resulted in a minimum necessary size of 68 patients per satisfaction group, 136 in total. This calculation also covered the study of the same objective for the complexity index of the concomitant medication. The nQuery Advisor 7.0 program was used for calculation.
The information collected was described after its statistical exploration. Quantitative variables were expressed with mean values and standard deviations, or median values and P25 & P75 percentiles in case of asymmetrical distributions, and qualitative variables through frequencies and percentages. In order to identify the complexity of HVC treatment as an indicator of dissatisfaction, Student’s t test was used for independent samples, or Mann-Whitney’s U test in case of non-normal distributions. The significant mean differences were quantified with 95% Confidence Intervals, and differences between medians with Hodges-Lehmann Confidence Intervals. For the research of associations of qualitative variables with yes/no satisfaction, the chi-Square test was used, or the non-asymptotic methods of Monte Carlo test and Exact Test.
Finally, there was an estimation of the relationship between total satisfaction and the Sustained Viral Response obtained with HVC treatment.
Data analysis was conduced with the IBM SPSS 23.0 statistical program for Windows.
The present study has been approved by the Ethics Committee of Research of South-Seville.
Results
The study included 171 patients in total (83.0% male), with a mean age of 52±8.7 years. The basal characteristics of the study population were shown in Table 1.
The mean score for satisfaction with treatment was 47.9± 7.54, with a mean of 24.92±3.66 scores for Overall Clinical Satisfaction, and a mean 19.07±3.61 for Satisfaction with Lifestyle. Table 2 shows the scores of the different items in the ESTAR adapted scale. 41.5% of patients presented high Overall Satisfaction (≥50 scores) with their treatment against the Hepatitis C virus.
The reliability of the complete questionnaire was high, with 0.864 Cronbach’s alpha (0.771 for the mean Overall Satisfaction, and 0.771 for the Satisfaction with Lifestyle), and a 0.843 Intraclass Coefficient (0.729 for Overall Satisfaction and 0.755 for Satisfaction with Lifestyle).
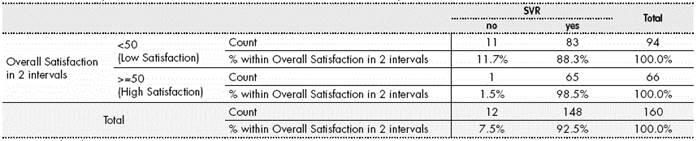
92.5% (n=148) of patients followed in the study achieved SVR vs. 7.5% (n=12) who did not reach it. It was not possible to obtain the SVR value for the rest of patients.
The statistical analysis demonstrated the correlation between the Complexity Index and Satisfaction (Table 3). The relationship between the complexity index of the treatment for HVC and satisfaction determined that a reduction in 5 scores in the complexity index of the treatment for Hepatitis C multiplied by four the value of Satisfaction with Treatment (p<0.0001). Similarly, 12 scores less in the Complexity Index of the Concomitant Medication allowed to double Patient Satisfaction (p=0.028).
Table 3 Correlation between the Complexity Index and Satisfaction according to the adapted ESTAR questionnaire
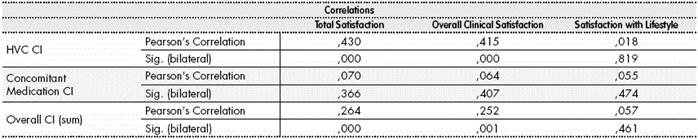
CI: Complexity Index: HVC: Hepatitis C; Sig level: significance (p<0.05) Pearson’s Correlation.
Regarding Overall Complexity, a value of 10 points less in this measure led to double the value of Perceived Satisfaction (p<0.05).
Finally, regarding the relationship between satisfaction and SVR obtained, those patients with higher satisfaction values presented a significantly higher percentage of SVR (0.029, Pearson’s Chi Square Test), as shown in Table 4.
Discussion
This study demonstrates that an increase in pharmacotherapeutical complexity is associated with lower satisfaction with treatment, both overall and therapy-specific.
Since the American Society of Hospital Pharmacists (ASHJP) issued their recommendations15 urging to conduct patients’ pharmacotherapeutical follow-up based on their pharmacotherapeutical complexity, some studies have been conducting assessments of this parameter and its outcomes in patient workload16, re-hospitalizations17, discrepancies in the administration of medication18 and treatment adherence19. As far as we know, this is the first study that associates this complexity with the outcomes perceived by patients, specifically with satisfaction.
To measure patient satisfaction represents a first-class objective, because it allows a direct knowledge of patients’ opinion about the services or treatments received; this is also considered a measure of direct health outcomes20. Concern for patients’ satisfaction is conditioned, because it is significantly and functionally associated with specific health behaviours (from adherence to prescriptions to follow-up of outcomes, or even actions to prevent the disease). In recent years, different tools have been developed to measure aspects associated with the overall quality of life of patients with Hepatitis C, such as HCV-PRO21 with high methodological consistency. However, it is difficult to implement it routinely in daily follow-up due to the complexity of its use. Similarly, we find the HCVT-sat tool22, easier to use and specific for satisfaction measurement, though not assessed in our health setting and developed in 2010, when the pharmacotherapeutical scenario was completely different to the current one.
Different studies have defined that the experience of patients is built on the basis of relational and functional aspects23-24. Some of the relational aspects of patient experience include emotional and psychological support, patient involvement in decision making, involvement by family and caregivers, clear information (and adequate for patient needs) and transparency. Among functional aspects, provision of effective services must be taken into account, as well as symptom management at the adequate time by competent professionals, the environment where care is provided, and care coordination and continuity. In this sense, it seems clear that the model to follow for the optimization of patients’ pharmacotherapeutical follow-up, in order to obtain better health outcomes and an improvement in patient satisfaction, includes an extension of the model for Selection and Stratification of Patients with Hepatitis C by the Spanish Society of Hospital Pharmacists (SEFH)25. This model allows to take into account not only the pharmacotherapeutical aspects associated with Hepatitis C, but also the complete pharmacotherapy and also clinical and emotional variables, and the use of healthcare resources. In this way, we could value not only satisfaction but also “the patient’s experience” or, in other words, the feedback by patients regarding what is happening throughout the process of care. We consider that in this way, this concept would become one of the cornerstones in the assessment of quality of care, at the same level as safety or efficacy, for which the new treatments for Hepatitis C are already reaching the highest levels. In other healthcare settings, the contribution by the pharmacist specialized in the management of Hepatitis C patients has also increased patient satisfaction, as demonstrated in the study by Martín et al26.
Finally, we should highlight the great relationship between the high satisfaction scores and the Sustained Viral Response obtained in the patients included in the study. Even though it is already known, and it has been studied for different conditions, that a higher satisfaction by patients is associated with better health outcomes, this is the first study that has demonstrated it for Hepatitis C, assessing the perspective of pharmacotherapy complexity.
This study presents various limitations; firstly, the ESTAR questionnaire has only been specifically validated for HIV patients. For this reason, strict reliability criteria were determined in order to assess the utility and feasibility of this questionnaire. Besides, the satisfaction with the concomitant treatment previous to the initiation of HVC treatment is unknown, because the majority of these patients have no interaction with the Hospital Pharmacy until their therapy against HVC is prescribed.
On the other hand, adherence to antiviral treatment was not assessed through specific questionnaires. However, given that pharmacotherapeutical follow-up was conducted on the basis of a model for selection and stratification of patients, and promoting the single act of care and with a model that includes its reinforcement, it was considered that the adherence estimated was necessarily high.
Finally, pharmacotherapeutical complexity was calculated at week 4 of treatment, and there might have been some variations throughout treatment evolution. However, given the differences in terms of duration, it was estimated that this was the most homogeneous point to learn about this relationship. There are no current studies assessing the percentage of variation in medical prescription, in terms of concomitant treatments, for this type of patients.
Future lines of research will allow us to understand if this satisfaction is sustained in patients with milder levels of fibrosis and, in theory, less motivated to receive treatment for their liver disease. Additionally, it would be necessary to know if, during the time on treatment for Hepatitis C, there could be a selective adherence to treatment and, consequently, lack of meeting the objectives for the concomitant chronic conditions in these patients, in case these are coexisting. Specifically, whether a higher or lower patient satisfaction is associated with fewer visits to the Emergency Unit, unscheduled visits, and higher use of resources for the self-management of the disease. Finally, those patients with low levels of satisfaction should be identified early, in order to act upon the factors causing it, and thus increase their likelihood of cure. In conclusion, an increase in pharmacotherapeutical complexity will have an impact on satisfaction with treatment, and at the same time, on the achievement of Sustained Viral Response in patients with Hepatitis C.











 text in
text in