Introduction
In the field of ophthalmology, there has been an increase in the use of blood derivatives for the treatment of ocular surface disease because their composition is analogous to natural tears1,2. The recent discovery of the role of platelets in tissue repair and regeneration has entailed a leap forward from the use of autologous serum to the application of so-called platelet-rich plasma (PRP)3.
Plasma rich in growth factors (PRGF) is a kind of these PRPs. Platelet-rich plasma is obtained from the patient’s blood and then it is activated with calcium chloride releasing a wide range of growth factors (GFs) and proteins involved in tissue regeneration4. In this way, an eye-drops enriched in growth factors and proteins is obtained. This has been used successfully in the treatment of several ocular surface diseases5-10.
On May 23, 2013, the Spanish Agency for Medicines and Health Products (AEMPS) published a resolution establishing the classification of the non-substitute therapeutic use of autologous plasma and its fractions, components, or derivatives as drugs for human use to attend special needs11.
The current classification of plasma derivatives as medicines for human use entails their fulfilling all the quality and safety requirements for ophthalmic drugs. In addition, the Good Practice Guidelines for the Preparation of Medicines in Hospital Pharmacy Services (Spanish: Guía de Buenas Prácticas de Preparación de Medicamentos en Servicios de Farmacia Hospitalaria) published by the Spanish Ministry of Health establishes that standardized preparations must be validated. This process entails the evaluation of the pharmaceutic properties of the preparation under real conditions from the first day of preparation to the expiration date.
The present study addressed the performance of an asepsis validation process when obtaining PRGF-Endoret® eye drops (BTI, Vitoria, Spain) using the PRGF-Endoret ® ophthalmology kit with culture medium. The study also evaluated the suitability of single-dose vials according to the requirements established in the Spanish Royal Pharmacopoeia on extractable volume of the eye drops vials and their tightness. The concentration levels of different GFs were also determined before and after sterilizing filtration of the PRGF eye drops. Furthermore, the effect of freezing/thawing on the biological potential of PRGF-Endoret® eye drops in cell cultures was also assessed.
Methods
The PRGF formulations used throughout the study were obtained in a private clinical setting. The study was conducted according to the principles of the Declaration of Helsinki (revised 2008) following approval by the corresponding ethical committee.
Sterility tests of the obtaining process of PRGF-Endoret® eye drops
Sterility tests were conducted according to the standards outlined in the European Pharmacopoeia. In summary, six PRGF-Endoret® ophthalmology kits were employed. Tryptica soy broth (TSB) was used as the culture medium, which substituted the patient’s blood throughout the PRGF- Endoret® eye drops preparation process. Three of the six kits were handled inside a laminar flow hood (1800 BBS-V, TDI, Spain) and the other three outside it. After the eye drops were obtained, the samples were incubated at 23 °C for the first seven days and at 33 °C for the following seven days. Finally, the content of each single dose was collected in a transparent test tube for its correct visualization.
To test microbiological growth, the culture medium was inoculated with 10 to 100 colony forming units using the following microorganisms:
- Staphylococcus aureus (ATCC 6538).
- Pseudomonas aeruginosa (ATCC 9027).
- Bacillus subtilis (ATCC 6633).
- Candida albicans (ATCC 10231).
- Aspergillus brasiliensis (ATCC 16404).
Bacteria and fungi were incubated for three days at 37 °C and five days at 23 °C, respectively.
Extractable volume test
To demonstrate that the nominal content of the single -dose vial could be extracted, the extractable volume test was applied to 5 PRGF-Endoret® eye drops vials using a different 1-mL dry syringe for each vial.
Single-dose vial tightness test
The tightness test was carried out by filling single-dose vials from two PRGF-Endoret® kits with water and placing them in a freezer for two days. Subsequently, they were thawed at room temperature for 4 hours (Figure 1A). The single-dose vials included in the rack of the kit were placed in a vacuum chamber, weighted down to avoid floating (Figure 1B), and immersed in toluidine blue solution (Figure 1C). A vacuum pressure of 0.65 bars was applied for 10 minutes (Figure 1D) and then each vial was carefully inspected. After this inspection, the single-dose vials were submerged without the rack (Figure 1E) in toluidine blue solution, weighed down (Figure 1F), and a vacuum pressure of 0.65 bars was again applied for 10 minutes (Figure 1G). Finally, the single-dose vials were again individually inspected.
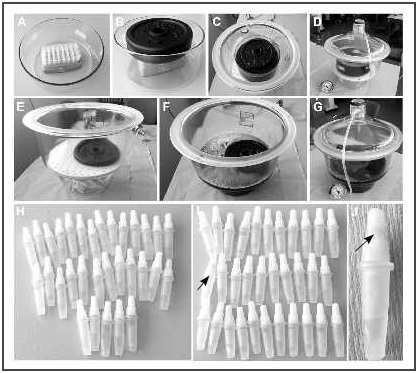
Figure 1. Tightness test. A, Single-dose vials in the rack in the vacuum chamber. B, Vials weighted down to prevent floating. C, Rack submerged in a toluidine blue solution. D, Sealing the vacuum chamber to apply vacuum. E, Single-dose vials without the rack. F, Submersed single-dose vials without the rack in toluidine blue solution with weight. G, Sealing and applying pressure. H, Detail of single-dose vials after the tightness test of the first rack. I, Detail of single-dose vials after the tightness test of the second rack. J, Detail of the cap with blue colouration.
Preparation of PRGF-Endoret® eye drops
The nature and potential consequences of the study were explained to healthy volunteers, who then gave their signed informed consent to participate. Blood samples were then extracted by venipuncture.
The PRGF-Endoret® ophthalmology kit was used, extracting blood into 9-mL tubes with 3.8% sodium citrate as anticoagulant. The blood samples were centrifuged at 580 g for 8 minutes and the complete plasma column was collected avoiding the buffy coat (leukocytes). Plasma was activated with 10% calcium chloride and incubated at 37 °C for one hour. Finally, the supernatant was filtered, aliquoted into single-dose dispensers, and frozen at -20 °C until use.
Analysis of growth factor concentrations
We measured the concentrations of the GFs involved in the regeneration of ocular surface tissues. These GFs included epidermal growth factor (EGF), platelet-derived growth factor AB (PDGF-AB), transforming growth
factor-beta 1 (TGF-ß1), vascular endothelial growth factor (VEGF), insulin-like growth factor type I (IGF- I), fibroblast growth factor (FGF), endostatin (END), angiopoietin-1 (ANG-1), and thrombospondin-1 (TSP-1). These GFs were analysed using commercial enzyme-linked immunosorbent assay (ELI-SA) kits (R&D Systems, Minneapolis, USA) according to the manufacturer’s instructions.
Cell cultures
The biological activity of the eye drop samples was studied in two types of primary cells from the ocular surface: human keratocytes (HK) and human conjunctival fibroblasts (HCF) (ScienCell Research Laboratories, San Diego, USA). Both cell types were cultured in fibroblast medium supplemented with fibroblast growth supplement (FGS), 2% foetal bovine serum, and the antibiotics penicillin and streptomycin (ScienCell Research Laboratories, San Diego, USA). Upon confluence, the cells were detached using a commercial enzyme free of animal traces (TrypLE Select, Gibco-Invitrogen, Grand Island, USA). Cell viability was analysed using the trypan blue exclusion staining method.
Cell proliferation
The ocular surface cells were seeded on a 96-well optical bottom black plates at 10,000 cells/cm2 and cultured for 72 hours using serum-free medium supplemented with the 20% PRGF-Endoret® eye drops obtained in each test. Cell culture density was analysed using the CYQUANT cell proliferation assay (Invitrogen, Carlsbad, USA) according to the manufacturer’s instructions. Sample fluorescence was measured using a fluorescence microplate reader (Twinkle LB 970, Berthold Technologies, Bad Wildbad, Germany). All quantifications included a DNA standard curve (ng/mL) to determine correlations between fluorescence units and DNA concentration.
Eye drop filtration test
Potential decreases in GF concentrations were determined by conducting an eye drop filtration test using a 0.22 μm PVDF filter (Millipore, Tullagreen, Ireland). Thus, half of the volume of the PRGF eye drops was aliquoted and frozen at -20 °C, whereas the other half was filtered using a 0.22 μm PVDF filter, and then aliquoted and stored at -20 °C until use. The concentrations of EGF, PDGF-AB, TGF-ß1, VEGF, FGF, END, and TSP-1 were measured in each sample.
Freeze/thaw cycle test
Filtered PRGF-Endoret® eye drops were used to assess the effect of a freeze/thaw cycle on their biological activity. The filtered eye drops were aliquoted and stored at -20 °C for two weeks. Subsequently, some of the aliquots were kept at -20 °C (control at -20 °C), whereas others were thawed at 4 °C (one cycle at 4 °C) or at room temperature (1 cycle at RT) for 18 hours. The aliquots of thawed eye drops were then refrozen at -20 °C and kept at this temperature until use. Subsequently, we analysed PDGF-AB, EGF, VEGF, TGF-ß1, ANG -1, END, and TSP-1 and the biological activity of the samples using HK and HCF proliferation assays. The concentration of ANG-1 was also measured, due to its greater sensitivity to the freeze/thaw process12.
Osmolarity and pH stability tests
Immediately after obtaining PRGF eye drops and after their storage for three months and six months at -20 °C, osmolarity was determined using the freezing-point depression method (Osmostat, OM -6020, Daiichi Kogaku Co., Kyoto, Japan), and pH was measured using a pH-meter (Crison Instruments, SA, Spain). All analytical determinations were conducted in triplicate.
Statistical analysis
Significant differences between eye drop samples were analysed using the Kruskal-Wallis nonparametric procedure followed by a Bonferroni test to compare differences between means. In the case of the stability test, significant differences between samples at two storage times were analysed using a nonparametric Wilcoxon test.
A p-value of < 0.05 was used as a cutoff for statistical significance. All statistical analyses were conducted using the SPSS software package (version 15.0, SPSS Inc., Chicago, USA).
Results
Sterility tests of the obtaining process of PRGF-Endoret® eye drops
The opthalmic dispensers were incubated at 25 °C and 33 °C. Following the incubation period, no turbidity, which is an indication of microbial growth, was detected in any of the 192 units analysed, regardless of whether they had been handled inside (n = 96) or outside (n = 96) the laminar flow hood.
In addition, the results of the microbiological growth used as a control, bacteriostasis and fungistasis tests, showed an increase in all strains from the second day of incubation.
Extractable volume
The extractable volume test showed that all the single-dose contained the nominal volume.
Vial tightness test
After applying the vacuum, there was no toluidine blue inside the vials or the caps of any of the 32 single-dose vials inside the rack (Figure 1H). Although there was no blue coloration in any of the vials without the rack, some coloration was detected inside of a vial cap (Figure 1I and Figure 1J).
Osmolarity and pH
There was a slight increase in osmolarity values from 321 ± 7 mOsm/L at the time of processing to 348 ± 3 mOsm/L and 360 ± 12 mOsm/L after three months and six months of storage at -20°C, respectively. Likewise, pH levels increased from 7.8 ± 0.1 at the time of processing to 8.10 ± 0.03 and 8.2 ± 0.4 after three months and six months of storage at -20°C, respectively.
Eye drop filtration test
The results of this test showed that there were no differences between the GF concentrations in the filtered eye drops (P > 0.05) and the unfiltered eye drops (Table 1).
Table 1. Growth factor concentrations measured in filtered and unfiltered PRGF-Endoret® eye drop samples*
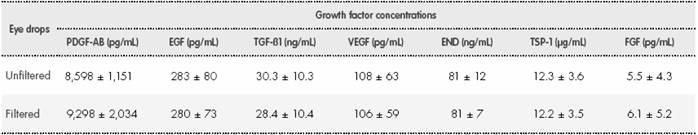
*No significant differences (P > 0.05) were observed in growth factor concentrations between any of the eye drop samples analysed. EGF: epidermal growth factor; END: endostatin; FGF: fibroblast growth factor; PDGF-AB: platelet-derived growth factor AB; TGF-ß1: transforming growth factor- beta 1; TSP-1: thrombospondin-1; VEGF: vascular endothelial growth factor.
Freeze/thaw cycle test
The results of this test showed that there were no significant changes (P > 0.05) in GF concentrations after the frozen eye drops had been thawed at 4 °C and at room temperature for 18 hours (Table 2) and after another frozen.
Table 2 Growth factor concentrations in the eye drop samples used in the thaw/freeze test*
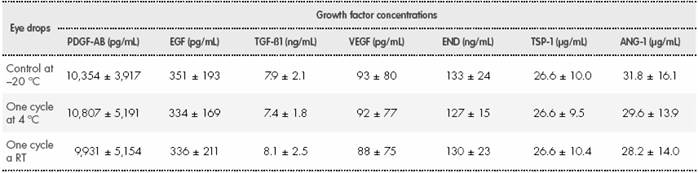
*No significant differences (P > 0.05) were observed in growth factor concentrations between any of the eye drop samples analysed. ANG-1: angiopoietin-1; EGF: epidermal growth factor; END: endostatin; PDGF-AB: platelet-derived growth factor AB; RT: room temperature; TGF-ß1: transforming growth factor-beta 1; TSP-1: thrombospondin-1; VEGF: vascular endothelial growth factor.
No changes were observed in the HK and HCF proliferation levels after treatment with PRGF eyedrops after one freeze/thaw cycle regarding to the control eye drops (Figure 2).
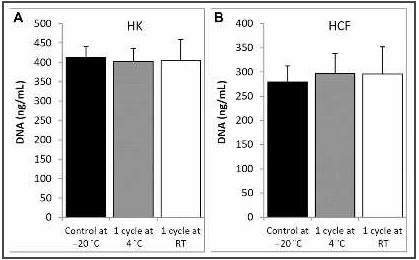
Figure 2. Ocular surface cell proliferation assay. A, Proliferation of HK cells. B, Proliferation of HCF. Response to PRGF-Endoret® eye drops at -20 °C (control at -20 °C), thawed at 4 °C (one cycle at 4 °C), and thawed at RT (one cycle at RT). HK: human keratocytes; DNA: deoxyribonucleic acid; HCF: human conjunctival fibroblasts; RT, room temperature.
Discussion
In 2013, the AEMPS issued a resolution establishing the classification of the non-substitute therapeutic use of autologous plasma and its fractions, components or derivatives as drugs for human use to meet special needs (http://www.aemps.gob.es/legislacion/espana/medicamentosUsoHumano/docs/medEspeciales/resolucion-PRP.pdf). This resolution was the first to regulate the use of PRP as a medicine for human use. This legal framework allows the use of PRP therapies while guaranteeing quality, efficacy, traceability, and pharmacovigilance. As with any other drug, minimum quality guarantees must be established throughout the manufacturing process. The Rules Governing Medicinal Products in the European Union (chapter 5) states as a general rule that products and materials must be protected from microbial contamination13 (https://www.aemps.gob.es/industria/inspeccionNCF/ guiaNCF/docs/reqBasicosMed/capitulo-5.pdf). In this regard, the results show that the process of obtaining the PRGF-Endoret® eye drops is aseptic, whether conducted inside or outside an laminar flow hood. Furthermore, the results of the tightness test showed that all the single- dose vials (100%) and almost all the caps (98.4%) were completely sealed.
Eye drops are the most commonly used solutions in the treatment and diagnosis of eye disease. To avoid adverse reactions, eye drops should have characteristics -such as being clear, isotonic, neutral, and sterile- that adapt to the physiological conditions of the eye. Blood-derived eye-drops typically undergo a final filtering process to achieve clarity14,15. The main drawback of this process is the potential loss of GFs due to their adhering to the filter. The results show that all the GF concentrations were preserved in the PRGF-Endoret® eye drops obtained after a filtering process with 0.22-μm PVDF filters.
Another key aspect of the eye drop production process is that they must be isotonic with natural tears. The physiological osmolarity of human tears ranges from 302 mOsm/L to 318 mOsm/L16,17. Healthy eyes can tolerate solutions with an osmotic pressure of up to 425 mOsm/L without pain or excessive tear production18. The results show that the osmolarity (321-360 mOsm/L) of the PRGF-Endoret® eye drops that had been frozen for six months was appropriate for ophthalmic administration.
Under normal conditions, tears have a pH of 7.4 to 7.7, although different diseases can modify their pH19. The buffer capacity of tears is enough to relatively quickly neutralize solutions over a wide range of pH values (3.5-10.5) providing the solutions are not buffered. The pH of the PRGF-Endoret® eye drops stored at -20°C for three months and six months remained within this range over the study period.
Regarding stability, the AEMPS report and the Spanish Good Practice Guidelines for the Preparation of Medicines in Hospital Pharmacy Services state that the stability of a drug must be known such that the quality guarantees can be fulfilled. A recent study has shown PRGF-Endoret® eye drops frozen for at least 6 months at -20°C and subsequently maintained for 72 hours refrigerated or at room temperature are biogically stable12. Microbiological stability must also be taken into account. As with all drug preparations, the Spanish Good Practice Guidelines for the Preparation of Medicines in Hospital Pharmacy Services should be applied. Thus, in the case of blood-derived eye drop preparations, the medication must remain in the production facilities for 7 to 14 days while awaiting the results of the microbiological tests. The eye drops must be stored at -20 °C. If the results of the microbiological tests are negative, the patients are given the eye drop vials and instructed to maintain the cold chain, otherwise the eye drops could thaw and then be refrozen when the patients arrive home. It is well known that freeze-thaw processes can degrade different proteins and GFs20,21, and thus this process could affect the biological activity of blood-derived eye drops. The results show that PRGF-Endoret® eye drops preserve intact the GFs analysed and their proliferative activity after a thaw/ refreeze cycle.
In summary, the PRGF-Endoret ® eye drops obtaining process is safe when following the guidelines on medicines for human use issued by the AEMPS and the Spanish Ministry of Health. In addition, the chemical and biological characteristics of PRGF-Endoret ® eye drops remain stable and at appropriate concentrations for their use in the setting of clinical ophthalmology.
Contribution to the scientific literature
Currently, there has been an increase in the incidence of ocular surface disease that requires treatment to maintain and regenerate the ocular surface. Autologous PRGF-Endoret® eye drops are an excellent alternative to the artificial tears in common use. These eye drops have to comply with a series of guidelines issued by the Spanish Agency for Medicines and Health Products and the Ministry of Health, to ensure their asepsis and safety as well as their chemical and biological properties over time after undergoing freeze/thaw cycles.











 text in
text in 


