Introduction
Hypercholesterolemia is both the most common lipid disorder and a cardiovascular risk factor1. Treatment comprises a combination of lifestyle changes and pharmacological treatment with statins, with or without other lipid-lowering drugs. In some patients, this treatment may be insufficient or they may develop intolerance2,3.
Proprotein convertase subtilisin/kexin type 9 (PCSK9) captures and eliminates circulating low-density lipoproteins (LDL). Anti-PCSK9 monoclonal antibodies2 prevent PCSK9 molecules from binding to LDL receptors, thus preventing a reduction in the number of LDL receptors on the hepatocyte surface. The use of the commercially available antibodies evolocumab4 and alirocumab5,6 is authorized in adults with primary hypercholesterolemia (non-familial hypercholesterolemia and heterozygous familial hypercholesterolemia) or primary and mixed dyslipidaemia. They can be used as monotherapy, in combination with a statin, with a statin and another hypolipidaemic agent, or in combination with other lipid-lowering agents in patients with intolerance or contraindications to statins.
Transplant patients are at increased risk of cardiovascular disease. Dyslipidaemias are among the causes of increased risk, and are strongly associated with the use of immunosuppressants. Similar to their treatment in the general population, these kinds of dyslipidaemias are treated with statins and other lipid-lowering drugs. In patients with intolerance or contraindications, the use of PCSK9 inhibitors has opened up a new treatment route. However, there are no specific studies on their effects in these patients.
Case description
45-year-old man, kidney transplant in July 2015, with well-controlled hypertension, hyperuricemia without gouty episodes, dyslipidaemia, history of hepatitis C virus (HCV) infection treated with ledipasvir/sofosbuvir in 2015 for 12 weeks with a successful response, and history of pulmonary embolism associated with immobilization due to a sports injury in 2010.
Initial immunosuppressive treatment comprised a combination of tacrolimus, mycophenolate mofetil, and prednisone, which is standard therapy in kidney transplant patients7. In April 2017, mycophenolate mofetil was replaced by everolimus because of intense generalized itching.
After the transplant, the patient’s lipid profile deteriorated. Treatment was started with high-dose statins (pitavastatin and subsequently atorvastatin) and ezetimibe. Due to liver function deterioration, therapy was suspended and a PCSK9 inhibitor (alirocumab 75 mg/15 d) was administered.
In September 2017, treatment was started with alirocumab. After two doses had been administered, he was admitted to the emergency unit with fever (39.9 °C), cough with yellowish expectoration, and odynophagia that lasted five days. He was admitted to the pulmonology unit with a diagnosis of retrocardiac pneumonia and sepsis of respiratory origin. After 5 hours he had hypotension, anuria, and compensated metabolic acidosis, and was transferred to the intensive care unit (ICU) for hemodynamic support. He remained in the ICU for 48 hours. After five days of admission, he was discharged from hospital, with alirocumab temporarily suspended.
Treatment with alirocumab was restarted after 90 days. Ten days later he contacted the nephrology unit because of a new cough with yellowish expectoration associated with low-grade fever and fatigue. Treatment was started with amoxicillin-clavulanate.
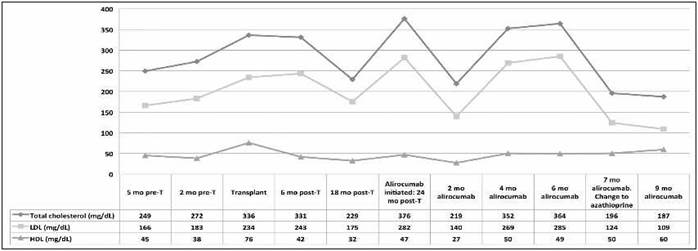
In February 2018, everolimus was replaced by azathioprine because of recurrent respiratory infection, severe hypercholesterolemia, and erythematous skin lesions. Simultaneous treatment with alirocumab was maintained. In May, the lipid profile (Figure 1) and cutaneous lesions improved and infectious respiratory symptoms did not recur.
Discussion
The main causes of mortality in kidney transplantation include cardiovascular complications, of which atherosclerosis and left ventricular hypertrophy are common8.
Dyslipidaemia in transplant patients is a common metabolic disorder of multifactorial aetiology and plays a prominent role in the development of atherosclerosis9. The following risk factors have been strongly associated with dyslipidaemia: immunosuppression, nephrotic syndrome, hypothyroidism, diabetes mellitus, excessive alcohol intake, obesity, chronic liver disease, genetic predisposition, and low physical activity.
The choice of immunosuppressants has a significant impact on dyslipidaemias. The most effective drugs include corticosteroids, calcineurin inhibitors (cyclosporine, tacrolimus), and mammalian target of rapamycin (mTOR) kinase inhibitors, such as sirolimus and everolimus. Mycophenolate mofetil and azathioprine play a less relevant role in dyslipidaemias.
Prior to transplant, the patient had high levels of cholesterol, which increased after the procedure. This increase could have been due to the administration of immunosuppressants, which is a well-known adverse effect, and to the reduction in physical activity over a period of months due to a necrosis in the right femoral head.
Given the patient’s high cholesterol levels, and in order to reduce cardiovascular risk, treatment was started with lipid-lowering agents (pitavastatin, atorvastatin, and ezetimibe). However, due to an adverse effect on liver function, these drugs were discontinued and therapy was started with the monoclonal antibody alirocumab.
Alirocumab5 is authorized in primary hypercholesterolemia and primary mixed dyslipidaemia. The Summary of Product Characteristics (SPC) for alirocumab describes oropharyngeal pain, rhinorrhoea, and sneezing as frequent adverse reactions in the upper respiratory tract. This SPC does not include risk of infection, whereas the SPC for evolocumab includes upper respiratory tract infections as a frequent adverse event. Transplant patients treated with immunosuppressants are at an increased risk of infection. Given that the addition of alirocumab may have increased the frequency and severity of infectious episodes, the PubMed and Embase databases were searched for studies or individual cases on the efficacy and safety of alirocumab in transplant patients. However, none were encountered.
It is noteworthy that shortly after starting treatment with alirocumab, the patient developed severe infectious processes, including a pneumonia episode that required his admission to the ICU. It should be emphasised that the infections occurred when alirocumab and everolimus were administered simultaneously. Although it is known that respiratory infections are more often associated with everolimus than with azathioprine10, potential interactions between everolimus and antibodies such as alirocumab or evolocumab remain unknown. After replacing everolimus with azathioprine, the respiratory symptoms did not recur, cholesterol levels decreased, and the patient’s general state improved. It was found that the lipid profile could be modified by changing the type of immunosuppressant and that the addition of the monoclonal antibody alirocumab increased the risk of infection. The risk/benefit ratio of the use of alirocumab should be reconsidered according to the type of immunosuppression used due to its potential influence on lipid profile and infectious risk. This ratio should be assessed periodically, target cholesterol levels established, and the drug should be suspended when these targets are reached. Alirocumab is a recently marketed drug that should receive maximum attention regarding the detection and reporting of adverse events, especially when used in patients whose characteristics differ from the study populations in different clinical trials. The Pharmacovigilance Centre of Asturias has been notified of this case.
Contribution to the scientific literature
Description of alirocumab-associated pneumonia, an adverse reaction not included in its SPC, but which is included in the SPC for evolocumab, which has a similar mechanism of action. Potential effect of immunosuppressants, although there are no studies on this topic.











 text in
text in