Introduction
In the last few years, immune checkpoint inhibitors (ICIs), anti-cytotoxic T-lymphocyte antigen (CTLA)-4 antibodies and, particularly, anti-programmed cell death-1 receptors (PD1) or their ligand (PD-L1), have been incorporated to the treatment of a variety of tumors, significantly extending survival in a number of patients1-3. On the other hand, the above-mentioned therapies are associated with a considerable toxicity profile4,5.
The huge economic and clinical impact of treatment with ICIs makes the need for further research and a thorough evaluation of the efficiency of these drugs all the more pressing. The main problem lies in the dearth of response-predictive biomarkers able to select those patients who will benefit from the therapy, which would reduce exposure to toxicity in those unlikely to benefit and save costs to the national health system.
Myriads of data are now available on the factors capable of influencing a subject's response to immunotherapy. These factors depend on the patient, the tumor, the environment and other variables including past and present treatments.
The main goal of studies in this field is to come up with immunotherapy response-predictive biomarkers that make it possible to select those patients with the greatest chance of obtaining a clinical benefit so as to maximize the risk-benefit ratio of immunotherapy regimens and reduce unnecessary costs.
The development of immunotherapy-specific biomarkers is a much greater challenge than the development of biomarkers for target-directed therapies. In the latter case, the search for biomarkers focuses on the specific characteristics of tumor cells and the driver mutations that occur in them. In the case of immunotherapy, apart from tumor cell alterations, other factors are involved, such as the characteristics of the tumor microenvironment and the host's immune response, which are equally or even more relevant6. Developing these biomarkers represents a significant challenge as it means working in a dynamic, more heterogeneous territory, where markers have a continuous range of expression and there is the added difficulty of having to establish cutoff points.
Against this background, the purpose of the present paper is to carry out a literature review aimed at identifying those tumor-dependent factors that play a role in regulating immune checkpoint inhibitors response, particularly those than may act as potential predictive biomarkers.
Methods
A structured review of articles included in the PubMed database was conducted with a view to identifying the tumor-related factors capable of influencing a subject's response to ICIs. The terms biomarkers, PD-1, PD-L1, CTLA-4, and checkpoint inhibitors were searched in the title and the abstract of a series of articles, including clinical trials, randomized clinical trials, reviews, systematic reviews and meta-analyses. Papers were required study human subjects; be written in either English or Spanish; and have been published between January 2015 and June 2019. The initial search was later expanded to include references in the selected articles that were considered relevant to the review.
Articles were selected independently by two of the authors. If an article got through the initial screening following a review of its title and abstract, the full text then perused to verify whether it was relevant to the aims of the study. In the event of repetition, articles with the highest degree of topicality, clarity and depth were selected. Extraction of data from the studies was also performed independently by the same two authors.
Results
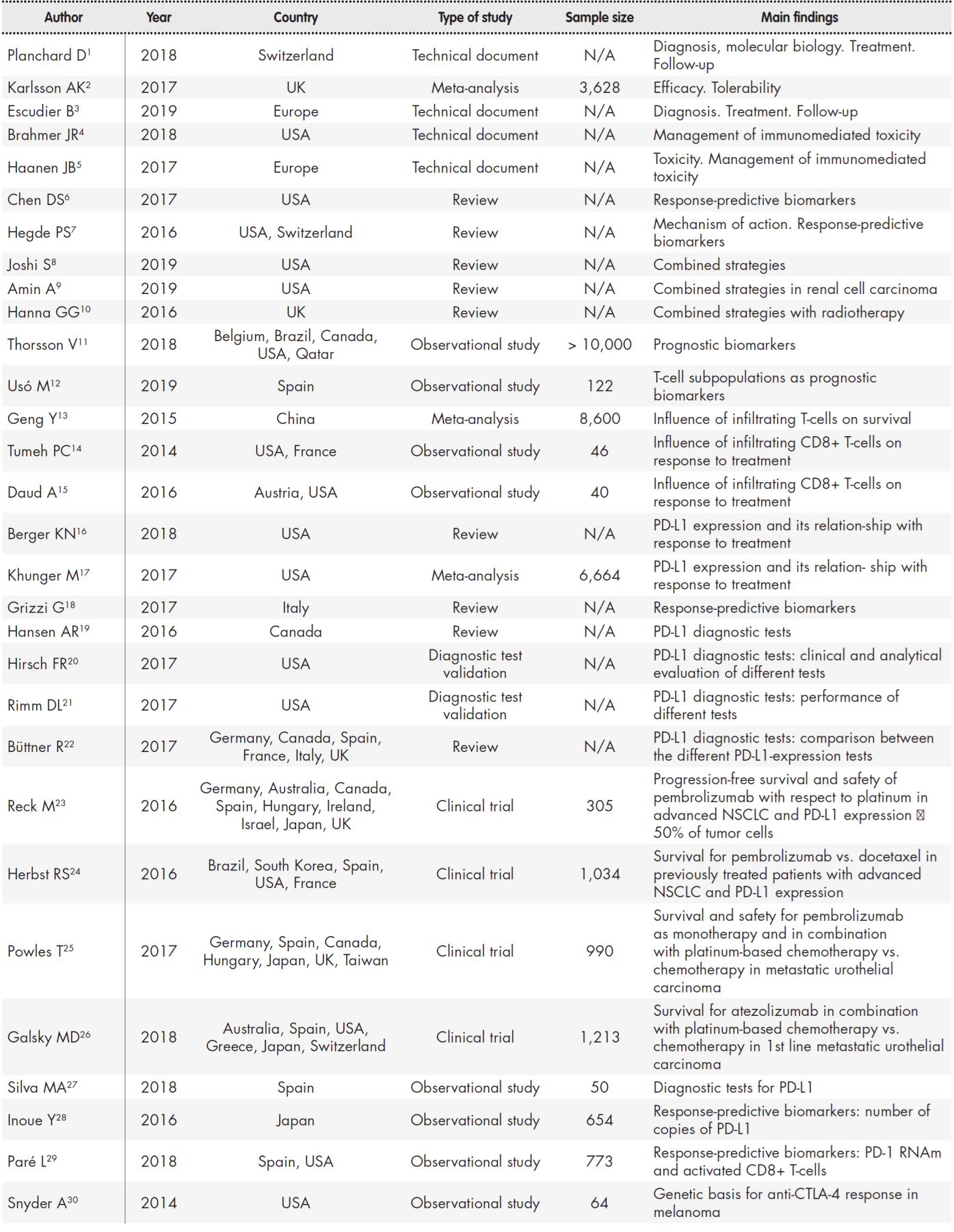
Fifty references were selected out of a total of 298 articles (31 clinical trials and 267 reviews) on account of their consistency with the purpose of the study (Table 1, Table 2).
Table 1 (cont.). Summary of studies included in the review
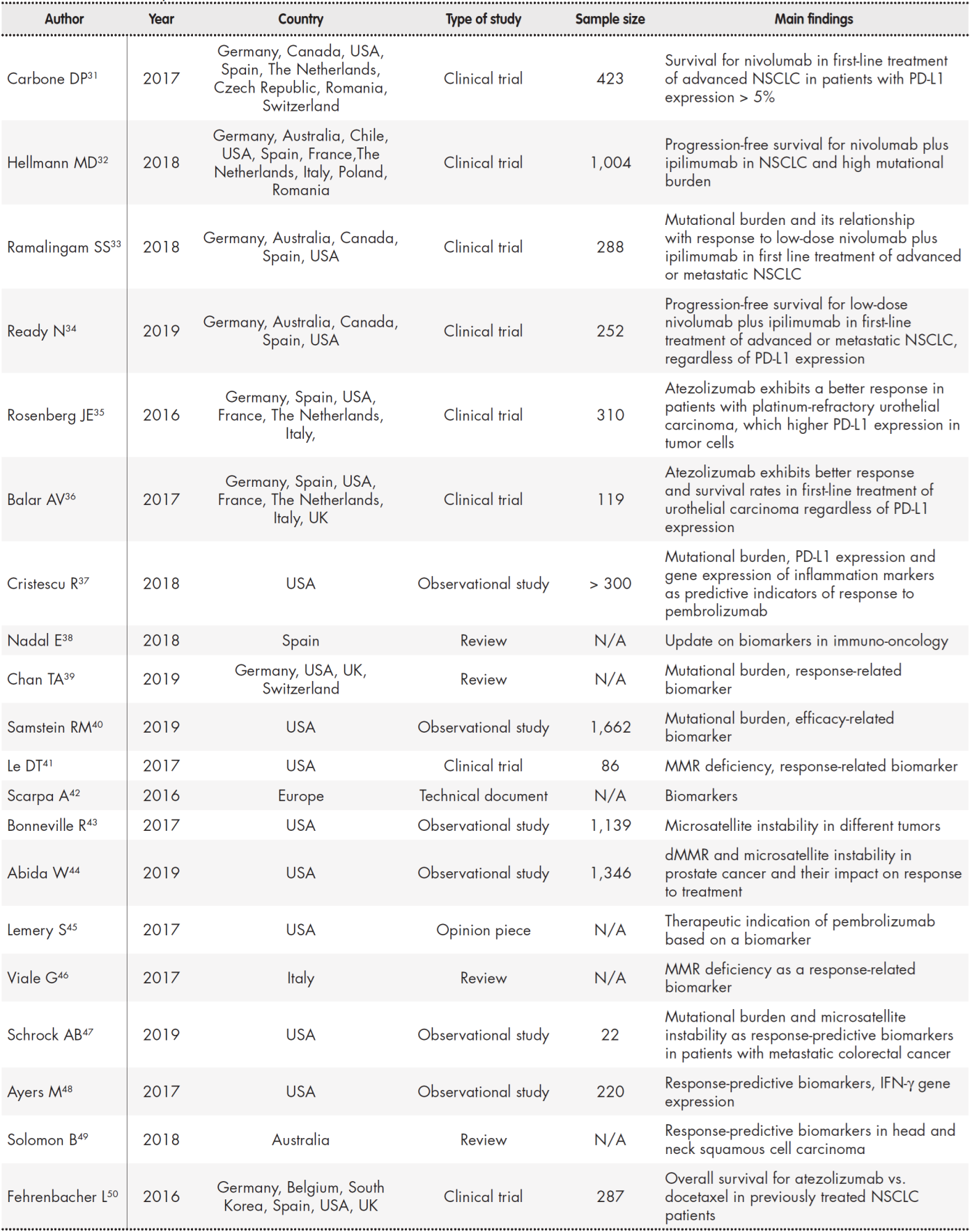
dMMR: deficient mismatch repair; IFN-γ: interferon-gamma; MMR: mismatch repair; N/A: not aplicable; NSCLC: non-small-cell lung cancer.
Tumor-related factors that have been associated with the efficacy or effectiveness of ICIs include those that determine the immune characteristics of the tumor's environment; the presence of certain tumor-infiltrating lymphocyte populations; the expression of PD-L1 by the tumor cells or the lymphocytes present in the tumor tissue; the tumor's mutational burden; or the shortage of DNA repair enzymes or microsatellite instability. In addition, gene signatures are being developed that may allow identification of tumors possessing these characteristics, which may make them more amenable to treatment with ICIs.
Immune profiles of tumor microenvironment: inmunophenotypes
Three immune profiles have been described in connection with the tumor microenvironment6,7, which are correlated with the subject's response to anti PD1/L1 therapy: a) the inflamed phenotype, which is characterized by infiltration by CD8+ cells and other immune cells (in the tumor parenchyma), accompanied by proinflammatory cytokines; b) the immune-desert phenotype, which does not contain an immune infiltrate and may result from tolerance mechanisms, immune ignorance, or the absence of T cell priming or activation; and c) the immune-excluded phenotype, where CD8+ T cells huddle around the tumor but do not infiltrate it. This may be due to the presence of barriers or vascular factors, or the presence of specific cytokines or inhibitory factors in the stroma.
A tumor located in an immune-desert or immune-excluded phenotype microenvironment is unlikely to respond to immunotherapy with ICIs. Although the inflamed phenotype is usually more likely to elicit a response, its presence does not guarantee that a response will be obtained. In fact, even if effector CD8+ T cells are infiltrated, such cells could be dysfunctional, with an exhausted or even hyperexhausted phenotype. In addition, the infiltrate may contain other immune-suppressive cells, such as regulatory T-cells (Treg), myeloid-derived suppressor cells, suppressor B lymphocytes or cancer-associated fibroblasts. Also, cells in these inflamed tumors may express inhibitory factors that reduce the expression of major histocompatibility complex (MHC) class I molecules (necessary for antigen presentation) or other pathways that decrease sensitivity to anti-tumor immunity6.
It must be noted that the previous or concomitant administration of other kinds of therapies (radiotherapy, chemotherapy, targeted therapy...) may result in inflammation of the tumor microenvironment, thereby enhancing sensitivity to ICIs. This has been the basis for the many combination studies currently underway8-10.
In April 2018, Thorsson et al.11 published the results of a complex immunogenomic analysis of over 10,000 tumors pertaining to 33 different types of cancer. Using data from the Cancer Genome Atlas project, the authors identified six immune subtypes as a function of the tumor microenvironment: wound healing, interferon-gamma (IFN-γ)-dominant, inflammatory, lymphocyte-depleted, immunologically quiet, and TGF- (transforming growth factor beta)-dominant. According to the authors, these immune subtypes have potential prognostic and therapeutic implications for cancer management.
Tumor-infiltrating lymphocytes (TILs)
Infiltration of a tumor by different kinds of immune cells has been associated with a significant prognostic value for different kinds of tumor, such as resected non-small-cell lung cancer (NSCLC)12) or metastatic NSCLC13.
Usó et al.12) described the relationship between infiltration by CD4+ and CD8+ cells in different tumor compartments and higher rates of progression-free survival (PFS) and overall survival (OS) in patients with resected NSCLC. Infiltration of the stromal compartment by FOXP3+ cells was associated with a poorer prognosis.
In a meta-analysis published in 2015, Geng et al.13 reported that deep infiltration of tumors by CD8+ and CD4+ T cells was often correlated with higher rates of OS in patients with metastatic lung cancer. Conversely, a high density of Treg lymphocytes (FOXP3+) may be interpreted as a negative prognostic factor.
Other authors have found a correlation between the presence of tumor-infiltrating CD8+ T cells and response to ICI therapy in other types of tumor. For example, in a paper published in Nature in 2014, Tumeh et al.14 reported that infiltration of CD8+ T cells into the invasive margin of the tumor can predict the response to anti PD1 treatment in patients with metastatic melanoma.
In 2016, Daud et al.15 went one step further and used multiparameter flow cytometry to analyze populations of tumor-infiltrating CD8+ T cells in patients with metastatic melanoma treated with nivolumab or pembrolizumab.
Patients with over 20% of cells with high CTLA4 and PD1 expression levels demonstrated a more favorable response to treatment than those with 20% or less. According to the authors, their data suggest that the relative abundance of partially exhausted CD8+ tumor-infiltrating lymphocytes can predict response to treatment with an anti-PD1 drug. Nonetheless, the technique used in their analysis is difficult to implement in clinical practice.
PD-L1 expression
The role of PD-L1 in the immune response to tumors can be summarized in the following way: the neoantigens expressed on the surface of cancer cells are recognized as extraneous, inducing a tumor-infiltrating lymphocyte-mediated antitumoral response. Part of that response involves the production of IFN-γ which, in turn, gives rise to the expression of PD-L1. The binding of PD-L1 to its receptor (PD1) inhibits the lymphocytes' effector functions16.
Expression of PD-L1 was the first response-predictive biomarker to be applied in clinical practice as has become a guide for the use of anti-PD1/PD-L1 drugs in certain clinical scenarios. PD-L1 expression is however an imperfect biomarker. Although a high expression of PD-L1 is generally correlated with a greater likelihood of obtaining a response or a clinical benefit17, a high expression does not in itself guarantee a response, nor is the absence of PD-L1 expression necessarily tantamount to a lack of response. This means that the level of PD-L1 expression cannot be used as a criterion to exclude patients from treatment with anti-PD1/PD-L1 drugs, except in very few cases, associated with the design of pivotal clinical trials. The biomarker's predictive value may also depend on the tumor's histology17.
The predictive value of PD-L1 expression has been studied at length in the realm of NSCLC, where it has been found to be associated with OS in patients treated with different PD1/PD-L1 inhibitors in monotherapy regimens18.
The clinical development and approval of the different compounds were based on the results of different diagnostic tests used to quantify PDL1 expresión19. These tests differed from one another in that they used different antibodies; they studied the marker in different cell types and tissue compartments; and they defined different positive staining thresholds. The use of different tests could result in patients being classified in accordance with disparate criteria and, therefore, in the inclusion of heterogeneous populations in different clinical trials, which would preclude indirect comparisons of the results of different studies. These limitations complicate patient selection in clinical practice.
Several authors have compared the different diagnostic tests
literature review published in 2017, Büttner et al.22 reported high inter-assay agreement and inter-observer reproducibility for three of the platforms available (when measurements were made in tumor cells) (28-8 PharmDx, 22C3 PharmDx and SP263 Assay). For immune cells, a higher level of intra-and inter-assay variability was found. The authors suggested that these three techniques could be interchangeable when used clinically in NSCLC patients. The SP142 Assay was associated with detection of lower levels of PD-L1, even in the tumor cell membranes.
Despite all the shortcomings, there are situations where determination of PD-L1 does influence patient selection. This is the case of pembrolizumab used as first-line therapy for NSCLC with PD-L1 > 50%23 and in the second line setting with PD-L1 > 1%24, as this was the inclusion criterion used in the clinical trials that provided these indications. In the context of urothelial cancer, in patients ineligible for platinum-based chemotherapy, initially was approved without PDL1 selection; but preliminary data from two phase III trials have shown lower survival rates when patients with low expression of PD-L1 were treated with pembrolizumab (Keynote 460)25 and atezolizumab (Mvigor 13026 as compared with patients treated with standard chemotherapy. As a result of these findings, both the FDA and the EMA have restricted the use of these drugs as monotherapy for patients with locally advanced or metastatic urothelial cancer who are cisplatin-ineligible and obtained a PD-L1 combined positive score ≥ 10% for pembrolizumab and ≥ 5% for atezolizumab.
Efforts are currently being made to look for alternatives to immunohistochemical tests for the determination of PD-L1 expression. For example, Silva et al.27 showed the advantages of differentiating between different staining patterns of reactive vs. constitutive PD-L1 expression to improve the biomarker's accuracy. Inoue et al.28, for their part, in a study of 654 patients with resected NSCLC, suggested an additional or alternative determination of the number of PD-L1 copies to improve the biomarker's predictive value.
Paré et al.29 established a connection between PD1 expression, measured by a determination of messenger RNA (nCounter platform), and OS and PFS in several tumor types. In this retrospective study, the authors analyzed paraffin-embedded samples of tumors treated with either nivolumab or pembrolizumab and found no correlation between the immunohistochemical determination of PD-L1 and the gene expression of PD1. The conclusion from this study was that the expression of PD1 is more strongly associated with sensitivity to anti-PD1 drugs than any other of the evaluated immune markers. PD1 expression could be associated to the presence of activated CD8+ T cells. It must be taken into consideration however that the subjects in this study were a heterogeneous group of patients with different kinds of tumors. Prospective randomized studies would be required to establish the clinical efficacy and the optimal positivity threshold of PD1 expression.
Tumor mutational burden
A study by Snyder et al.30, published in 2014, was one of the first analyses to provide tumor mutational burden (TMB) data, based on a whole exome analysis, in patients with metastatic melanoma treated with ipilimumab. These authors found a statistically significant difference in mutational burden between patients obtaining a long-standing clinical benefit (> 6 months) and those with minimal or no clinical benefit. It must be said that no gene was found to be universally mutated in patients showing a sustained clinical benefit. These authors also classified patients into those with a high number of mutations in the exome (> 100 mutations) and those with a lower number and observed statistically significant differences between the OS curves for these two groups of patients. These results made the authors propose TMB as a predictive biomarker capable of identifying immunotherapy-eligible patients. The underlying mechanism proposed is based on a connection between a high mutational burden and an increased number of neoantigens which, on being recognized as extraneous by the T-cells would become more immunogenic and trigger an anti-tumor immune response.
In 2017, the CheckMate 026 trial31 showed that nivolumab was not superior to chemotherapy in first-line treatment of (squamous and non-squamous) NSCLC, in patients with > 5% PD-L1 expression (primary endpoint), or in patients with > 1% PD-L1 expression (secondary endpoint). The authors performed an exploratory (not pre-specified) subgroup analysis comparing PFS results as a function of TMB. They carried out a whole exome analysis where the mutational burden was considered high if the number of mutations was > 243. In patients with high TMB levels, PFS is longer in the nivolumab group, while patients with low or moderate TMB levels showed a longer PFS with chemotherapy.
CheckMate 227, the first phase III trial to use TMB for patient selection, demonstrated the usefulness of this biomarker in a prospective way32. This trial focuses on first-line treatment of NSCLC and compares three different therapies: ipilimumab plus nivolumab, chemotherapy, and nivolumab as monotherapy. Results show that PFS in patients with high TMB is significantly higher with the nivolumab-ipilimumab combination than with chemotherapy, regardless of the tumor's histology (squamous vs. non-squamous) or PD-L1 expression (≥ 1% vs. < 1%). This analysis determined TMB through a gene panel, using the FoundationOne CDx platform (Foundation Medicine, Cambridge, Massachusetts, USA). TMB was considered elevated if the number of mutations per megabase was
> 10, based on the findings of the phase II CheckMate 568 trial33,34. This trial, which evaluated the efficacy of nivolumab plus ipilimumab in NSCLC patients, found that the 10 mutations mark was an effective cutoff point to select the patients who are most likely to respond, regardless of their tumor's PD-L1 expression.
The connection between TMB and therapeutic efficacy has been observed in other clinical situations, e.g. the use of atezolizumab to treat urothelial carcinoma35,36, or the use of pembrolizumab to treat a variety of other tumors37.
The advantage of using TMB as a predictive biomarker is that it is a quantitative and more reproducible indicator than the immunohistochemical tests used to determine PD-L1 expression. However, it is also associated with a few disadvantages. Firstly, the different trials currently underway to investigate the efficacy of TMB as a biomarker apply disparate methodologies and a different terminology. Moreover, there is no consistency in the positivity thresholds used to determine whether a patient has a high, moderate or low TMB level38,39; and cutoff points may be different for different types of tumors40. Finally, a molecular analysis of the samples in the CheckMate 227 trial has shown that only 58% of paraffin-embedded samples were eligible for TMB analysis, which raises doubts regarding the possibility of applying these approaches to clinical practice.
Impairment of DNA repair genes/microsatellite instability
Tumors exhibiting deficient mismatch repair (dMMR) during DNA replication present with an exceptionally large amount of somatic mutations in their genome. The data resulting from the phase II trials conducted by Le et al.41 support the hypothesis that a high load of neoantigens arising from mutations in cancers displaying dMMR makes such cancers sensitive to immune checkpoint inhibition, regardless of the nature of the original tumor tissue.
These authors evaluated the efficacy of PD1 inhibition with pembrolizumab in 12 different types of tumors presenting with dMMR. The analysis yielded an objective response rate of 53%, with 21% of complete responses. Estimated 12-and 24-month PFS was 64% and 53%, respectively; and estimated OS after the same two periods was 76% and 64%, respectively. These results are better than expected, taking into consideration the advanced stage of the disease in the patients included in the cohort. The analysis showed no significant differences between patients with colorectal cancer and other patients, or between patients with or without Lynch syndrome. The authors also calculated the percentage of tumors with dMMR in a sample of 12,000 tumors pertaining to 32 different histological types: dMMR was detected in over 2% of endometrium, stomach, small intestine, colon and rectum, cervix, prostate, bile duct and liver adenocarcinomas, as well as in neuroendocrine tumors, non-epithelial ovarian cancer and uterine sarcoma. Given the spectacular results they obtained with anti-PD1 drugs, the authors recommended the performance of dMMR tests in all patients refractory to standard treatment to identify those who could benefit from PD1 inhibition, regardless of their tumor type.
Microsatellites are repetitive nucleotide sequences distributed throughout the genome. These repetitive sequences are highly prone to mutations, which are generally repaired by error-correcting genes. When such genes are inactivated, a phenomenon known as microsatellite instability (MSI) occurs. Identification of MSI by a molecular test is a direct proof of the presence of dMMR42.
Microsatellite instability/dMMR has been described in many solid tumors, sometimes in high proportions. Such is the case of endometrial carcinoma (33%), gastric and colorectal tumors (15%) and ovarian and duodenal cancer (10%)42. Bonneville et al. identified MSI in 3.8% of over 11,000 samples of 27 different types of tumors (obtained from the Cancer Genome Atlas project), and described the phenomenon even in more uncommon tumors such as adrenocortical carcinoma or mesothelioma43.
Abida et al. found a 3.1% prevalence of MSI in 1,033 patients with prostate cancer and reported that, in some cases, the molecular phenotype was acquired in the course of the development of the disease. Eleven patients with MSI/dMMR were treated with PD1/L1 inhibitors, with promising responses44.
Use of MSI/dMMR as a predictive biomarker has been incorporated to clinical practice in some specific scenarios. The FDA approved nivolumab for colorectal cancer with MSI in 2017 and more recently approved pembrolizumab, the first drug authorized for use based on a molecular biomarker, regardless of tumour type45.
Clinical trials are underway on ICIs (on their own and in combination) to explore the predictive role that the presence of dMMR/MSI may play in different solid tumors46. A recent study has found TMB to be associated with an additional predictive value in colorectal cancer patients with MSI47.
Gene signatures or inflammation signatures
Several signatures or platforms of gene expression are being developed, made up of genes associated with the activation of IFN-γ (a T-cell activation mediator). Such platforms, also known as inflammation signatures, are used to carry out a transcriptomic analysis of the expression of genes that may cause the activation of T cells.
In an analysis of gene expression profiles using RNA, from baseline samples obtained from tumors of different etiologies treated with pembrolizumab, Ayers et al.48 identified a signature of 18 genes associated with IFN-γ production and T-cell activation, which were correlated with the clinical benefit obtained from the treatment. The results of their pilot study, which analyzed samples from patients with melanoma, were confirmed in a larger cohort of patients with melanoma and other cohorts of patients with solid tumors of different origins such as head and neck49 and the GI tract. According to the authors, their results demonstrate that an inflamed microenvironment (characterized by active signs of IFN-γ, cytotoxic effector molecules, antigen presentation and T cell activating cytokines) is a biological hallmark of ICI treatment-responsive tumors. According to these authors, a small group of genes could identify this biological profile and predict a subject's clinical response of a wide variety of tumors.
In 2016, Fehrenbacher et al.50 published the results of the POPLAR trial, a phase II study comparing atezolizumab with docetaxel in previously-treated NSCLC patients. As part of an exploratory analysis of biomarkers, these authors evaluated their results as a function of a signature of genes associated with T-cell activation, immune cytolytic activity and IFN-γ expression. They found that patients with high levels of that activation signature exhibited a better response to treatment with atezolizumab, with higher OS. However, in patients undergoing chemotherapy, having a high or a low level of the signature had no impact on the outcome of the treatment. It must be added that a high expression of this gene signature was associated with PD-L1 expression in tumor-infiltrating immune cells but not in tumor cells.
This signature, which at the beginning included the expression of around 18 different genes, was subsequently restricted to just three genes (PD-L1, IFN-γ and CXCL9), and was shown to be capable of successfully identifying those patients who would benefit from treatment with atezolizumab.
Discussion
The advent of ICIs has made it possible to treat several types of tumor in a more accurate and individualized manner. It is now a priority to understand factors capable of influencing the response and to develop predictive biomarkers to identify those patients that are most likely to benefit from ICIs. The present review is not exempt from limitations given the difficulties inherent in interpreting the results of the different studies, many of them retrospective and presenting preliminary data, and the variability of the clinical scenarios analyzed. Implications for clinical practice are for now limited to the use of PD-L1 and MSI as biomarkers in some specific situations. A systematic survey of accessible factors and of the results obtained in clinical practice may contribute to improving patient selection.
In conclusion, the complexity of the relationship between the immune system and tumour means that the tumoral factors capable of predicting a subject's response to ICIs are extremely varied and scarcely understood. This complicates the development of simple and/or universal predictive biomarkers. The only biomarkers used in current clinical practice are PD-L1 expression and MSI/dMMR, albeit with limited utility. TMB and IFN-γ-associated gene signatures may potentially become useful biomarkers once the determination techniques have been systematized and the required cutoff points have been established.











 text in
text in