My SciELO
Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista de la Sociedad Española del Dolor
Print version ISSN 1134-8046
Rev. Soc. Esp. Dolor vol.23 n.1 Madrid Jan./Feb. 2016
ORIGINAL
Tapentadol in the management of opioid-naïve patients with cancer pain
Tapentadol en el manejo del dolor oncológico en pacientes libres de opioides
E. López Ramírez, D. M. Muñoz Carmona1, J. Contreras Martínez2 and A. de la Torre-Luque3
Radiation Oncology Department. ONCOSUR. Granada.
1Radiation Oncology Department. H. U. Juan Ramón Jiménez. Huelva.
2Radiation Oncology Department. H. U. Carlos Haya. Málaga. Personality, Assessment and Psychological Treatment Department. Granada, University. Spain
ABSTRACT
Introduction: Tapentadol is a centrally acting analgesic with two mechanisms of action (μ opioid agonism and norepinephrine reuptake inhibition).
Patients and methods: Tapentadol in 53 cancer opioid-naïve patients with chronic and/or acute pain treated with tapentadol in 3 Radiotherapy Departments from October 2011 to February 2013.
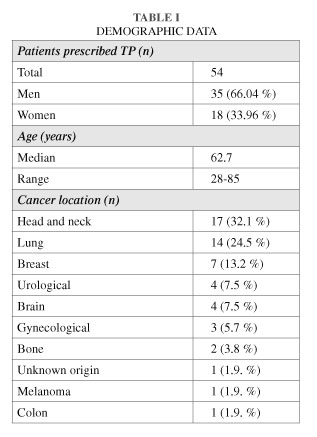
Results: Patients included 18 women (33.96 %) and 35 men (66.04 %) aged 28-85 years (mean: 62.7). Treatment was suspended due to death in 16.98 %, improvement in 5.66 %, pruritus in 1.9 % and dizziness in 1.9 %. Treatment was continued in 66.03 %, and doses increased in 26.41 % to achieve analgesia while 7.5 % were switched to another drug.
a) The most common cancers were head and neck in 32.1 %, lung in 24.5 % and breast in 13.2 %.
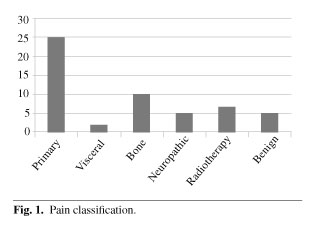
b) Pain was due to: 47.16 % tumor, 18.7 % bone metastases, 13.21 % radiation therapy, 7.55 % benign processes, 7.55 % neuropathic pain and 3.77 % visceral metastases.
c) Visual Analog Scale pain pre-treatment was 7.2 and post-treatment 3.3 (difference: 3.9 points), while 71.8 % progressed to mild pain (VAS ≤ 4).
d) The dose most used was: 50 mg (50.9 %).
e) Associated medications were: none (22.64 %), rapid-onset fentanyl (60.38 %), anticonvulsants (17 %), steroids (17 %), NSAIDs (13.2 %), morphine (5.66 %), anxiolytics (1.9 %), antidepressants (1.9 %), lidocaine 5 % (1.9 %) and acupuncture (1.9 %).
f) Analgesic efficacy was achieved in 94.34 % of cases. Mean analgesia was reached by 58 % of patients and maximum analgesia was 87.5 % in one patient.
g) Tapentadol was well tolerated with mild side effects (pruritus, constipation and dizziness) in 4 cases (10.7 %).
Conclusions: Our data support the use of Tapentadol in cancer opioid-naïve patients with moderate-to-severe chronic or acute pain (VAS > 5). Tapentadol is an effective pain reliever with few side effects.
Key words: Cancer pain, analgesic, opioid, norepinephrine, tapentadol.
RESUMEN
Introducción: El tapentadol es un analgésico de acción central con dos mecanismos de acción (agonismo µ opioide e inhibición de la recaptación de norepinefrina).
Pacientes y métodos: Desde octubre de 2011 a febrero de 2013 hemos realizado un estudio prospectivo de cohorte observacional para evaluar la eficacia del tapentadol en 53 pacientes oncológicos libres de opioides con dolor crónico o agudo en tres Servicios de Oncología Radioterápica.
Resultados: Los pacientes fueron 18 mujeres (33,96 %) y 35 hombres (66,04 %) con una edad entre 28-85 años (media: 62,7). El tratamiento se suspendió por fallecimiento en el 16,98 %, por mejoría del dolor en el 5,66 %, por prurito en el 1,9 % y por mareo en el 1,9 %. El tratamiento se mantuvo en el 66,03 % y las dosis se aumentaron para alcanzar la analgesia en el 26,41 %, mientras que en el 7,5 % se rotó a otro fármaco.
a) Los cánceres más comunes fueron de cabeza y cuello en el 32,1 %, pulmón en el 24,5 % y mama en el 13,2 %.
b) El dolor era debido al tumor en el 47,16 % de los casos, metástasis óseas en el 18,7 %, radioterapia en el 13,21 %, dolor neuropático en el 7,55 %, otro proceso benigno en el 7,55 % y metástasis viscerales en el 3,77 %.
c) La Escala Visual Analógica (EVA) pre-tratamiento era de 7,2 y post-tratamiento de 3,3 (diferencia de 3,9 puntos). El 71,8 % de los pacientes evolucionó a un dolor moderado (EVA ≤ 4).
d) La dosis más utilizada fue de 50 mg (50,9 %).
e) Otras medicaciones asociadas fueron: ninguna (22,64 %), fentanilo de liberación rápida (60,38 %), anticonvulsivantes (17 %), esteroides (17 %), antiinflamatorios (13,2 %), morfina (5,66 %), ansiolíticos (1,9 %), antidepresivos (1,9 %), lidocaína 5 % (1,9 %) y acupuntura (1,9 %).
f) La eficacia analgésica se alcanzó en el 94,34 % de los casos. Una analgesia media se consiguió en el 58 % de los pacientes y una máxima del 87,5 % en un paciente.
g) El tapentadol fue bien tolerado con moderados efectos secundarios (prurito, estreñimiento y mareo) en 4 casos (10,7 %).
Conclusiones: Nuestros datos apoyan el uso del tapentadol en los pacientes con cáncer libres de opioides con dolor crónico moderado-severo o agudo (EVA > 5). El tapentadol es un analgésico con pocos efectos secundarios.
Palabras clave: Dolor oncológico, analgesia, opioides, norepinefrina, tapentadol.
Introduction
Owing to the increasing incidence of cancer, cancer-related pain is a major public health problem worldwide (1-3). Meta-analyses revealed a 50 % prevalence of pain in the cancer population (4), and over 90 % of cancer patients experience cancer pain throughout their disease (5).
Unfortunately, available options for the successful treatment of cancer pain are still massively underutilized by physicians, and many patients suffer from insufficiently controlled pain despite available treatment options (1,6).
Appropriate management of pain remains a considerable challenge for health care providers. As the main reasons for these findings, Breuer et al. (6) reported a lack of information of the physicians and a limited willingness to integrate specialized services in the trajectory of cancer care.
In addition, prolonged acute pain can cause sensitization of the central and peripheral nervous systems, leading to the development of chronic pain, which is often difficult and costly to treat (7-10).
New analgesics have been developed with the purpose to improve the pharmacological profile of opioids, by reducing adverse effects (11).
Tapentadol (TP) extended release (ER) is a novel, centrally acting analgesic that offers analgesic efficacy similar to that provided by pure µ-opioid agonist comparators and norepinephrine reuptake inhibition, with an improved side effect profile, and may represent a significant advancement in the management of moderate-to-severe acute pain (7,12,13).
In non-malignant conditions, the efficacy and safety of TP ER in humans have been demonstrated in several comparative studies with placebo and oxycodone (7,14,15).
In cancer conditions in preclinical studies, a recent experimental analysis of tapentadol on spinal neuronal signalling in a rat model of metastatic bone pain, has found a marked inhibition of the neuronal activity with efficacy against mechanical, thermal and electrically evoked activity following tapentadol administration. In addition, the effects of the drug were fully reversible by naloxone and partly by atipamezole, supporting the idea of MOR-NRI dual actions (16).
The efficacy and safety of tapentadol ER for the management of moderate to severe, chronic tumor-relate pain have been demonstrated in phase III clinical studies demonstrating that tapentadol provides comparable efficacy to that of morphine sulfate CR but it is associated to better gastrointestinal tolerability (17). These results are also supported by a study performed in Japanese and Korean patients with moderate to severe, chronic malignant tumor-related pain (18).
In clinical practice, the drug might be of interest, particularly in patients who might have some element of hyperalgesia associated with prolonged treatment with opioids at relatively high doses (13). Also in metastatic bone patients because bone pain has been associated with a masked state of hyperalgesia (19). Thus, tapentadol is a therapeutic alternative for cancer patients with metastatic pain complication.
Finally it is important to pointed out that in opioid-naïve patients with moderate-severe pain we can argue two reasons: 1) TP ER needs low escalation indexes than patients with morhphine (20), transdermal buprenorphine (21) and transdermal fentanyl (22); 2) The percentage of patients who discontinued TP (7 %) seems to be even less than patients treated with oral morphine (13 %), transdermal buprenorphine (15 %), and transdermal fentanyl (about 14 %) reported in previous studies with a similar design and duration (20-23). So, TP may be of particular benefit o opioid-naïve patients or, for example, in the elderly (23).
The aim of our study was to evaluate the effectiveness and tolerability of TP ER in our series of cancer opioid-naïve patients with chronic and/or acute pain.
Patients and methods
We conducted a prospective observational cohort study in which 53 cancer patients were asked about their pain and its characteristics at their visit to a Radiotherapy and Oncology Department. The majority of patients were opioid-naïve cancer patients (n = 50). Three different Radiotherapy and Oncology Departments participated in the study. Pain evaluation was performed using the Visual Analog Scale (VAS). Patients with a VAS >5 were prescribed TP ER.
During the period of October 2011-February 2013, 53 patients were prescribed TP ER.
The majority of patients initially received twice-daily doses of TP ER 50 mg while 3 patients received 25 mg/12 h. Doses were managed to maintain adequate relief or dose-limiting toxicity on the basis of clinical response.
We collected information relating to TP ER including pain intensity pre- and post-TP ER, type of pain, drug combinations, TP ER therapy doses, efficacy, and side effects.
The classification of analgesic response to TP is a modification of the analgesic response proposed by the International Bone Metastases Consensus Working Party (24). The degrees of response have been defined as follows:
- Potent response was defined as pain reduction of four or more points in the VAS score with no concomitant increase in analgesic intake.
- Partial response was defined as pain reduction of two or more points in the VAS score with no analgesic increase or an analgesic reduction of 25 % or more from baseline with no increase in pain.
- Progression/no response was defined as an increase in two or more points in the VAS score above baseline with stable analgesic use or an increase of 25 % or more with a pain score that was stable or one point above baseline.
Statistical analysis was performed with SPSS 19.0. The paired sample Student's t-test was used to compare VAS pre- and post-TP ER treatment. We used a chi-square test to compare patients requiring and not requiring increased TP ER doses. To compare the percentage of patients requiring TP ER dose escalation or not we used a chi-square independence test.
Results
During the period from October 2010 to February 2013 we treated 53 patients for cancer pain with TP ER in three different Radiotherapy and Oncology Departments. All patients were referred to our Departments due to various pathologies requiring radiotherapy. Demographic and tumor data of treated patients (n = 53) are shown in Table I.
The majority of patients had locally advanced (26 %) or metastatic disease (60 %).
In our series 47.16 % of patients (n = 25) presented with pain caused by the tumor, 18.87 % (n = 10) by bone metastases, 13.21 % (n = 7) by radiation therapy, 9.43 % (n = 5) by benign processes, 9.43 % (n = 5) by neuropathic pain, and 3.77 % (n = 2) pain from visceral metastases (Figure 1).
A total of 94.34 % were opioid-naïve cancer patients. Three patients (5.66 %) had previously received opioid treatments (morphine or oxycodone) before TP ER without achieving adequate analgesia.
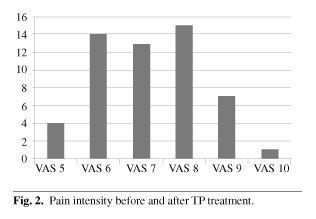
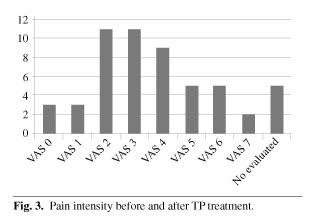
Pain intensity before TP ER treatment was VAS > 5 in all cases. Forty one patients (77.35 %) had VAS 6-8. Pain intensity after TP ER treatment was VAS < 4 in 38 patients (71.67 %) (Figures 2 and 3). We can consider that this analgesic effect was potent. In 50.9 % of patients pain was controlled with a TP ER dose of 50 mg/12 h (relatively low). This suggests slow development of tolerance.
The analgesic effect of TP ER was potent in 35 cases (66.03 %), partial in 14 cases (26.41 %), and absent (no response) in 4 cases (7.5 %) that were switched to morphine (2) or fentanyl (2).
The primary effect of TP treatment was in subjective pain level, with a t (49) of 19.33, p < 0.001, d = 2.55.
Patients with higher VAS pre TP ER treatment did not require either higher doses of TP ER or dose escalation to reach a good level of analgesia (p > 0.05).
TP ER dose was maintained in 66.03 % of the patients, which supports the idea that TP ER has a slow development of tolerance X2 (1) = 8.00, p < 0.05.
We prescribed 32 treatments with TP ER plus Rapid Onset Opioids (ROO); 12 patients did not need an associated drug; 9 patients received corticosteroids; 9 anticonvulsants; 7 patients required anti-inflammatory agents; 3 treatment with sulfate of morphine; and 4 patients other drugs (benzodiazepines, antidepressants, lidocaine patches and acupuncture).
The only adverse effects reported following TP ER treatment were described by 4 patients (7.55 %), including pruritus (1), constipation (2) and dizziness (1). None of the patients reported any severe effects.
Reasons for stopping TP ER therapy were ineffective treatment in 4 patients, side effects in 2 patients, and cure of pain in 3 patients. Nine patients died during the study period.
Discussion
Despite the recognized importance of cancer pain management, a substantial proportion of patients with cancer pain (approximately 10-30 %) do not achieve an adequate balance of pain relief and tolerability using systemic analgesics, often due to the occurrence of opioid-related adverse effects, which prevent patients from reaching the dose that would provide optimal analgesia (25). As many as 40 % of patiens with cancer without previous vomiting, experience opioid-induced nausea and vomiting (26).
TP ER is a newly developed synthetic opioid that was originally developed for the management of moderate and severe chronic non-cancer pain (27). Aside from its agonistic effect at the μ-opioid receptor, it inhibits central norepinephrine reuptake (27).
Due to these unique features it was assumed that the use of the substance would be associated with a reduction in opioid-related adverse effects but with equivalent analgesic potency to typical μ-agonists (12). Publications studying the use of TP in various pain models have reported that the development of opioid tolerance was considerably delayed compared with other opioids (14,23,28,29).
Opioid agonism reduces spinal pain transmission and show activity at supraspinal sites through descending projections that further reduce sensory transmission. The inhibition produced by norepinephrine enhancement may activate descending inhibition of pain transmission, probably via α2-adrenoreceptors (12).
The norepinephrine inhibition appears to predominantly mediate antihypersensitive effects rather than antinociceptive effects. The latter may be more affected by the opioid agonist activity of TP ER (23,30). Norepinephrine inhibitory mechanism may be less prone to the development of tolerance than µ-opioid agonism (23,31) and may add antihyperalgesic effects (23,30).
Cancer patients, in contrast to non-cancer patients with pain, may require high doses of opioids due to disease-related factors, pain characteristics, or prolonged use. Thus, it is of paramount importance to gather information about the use of this new drug with its unusual pharmacologic characteristics in this context (23).
Mercadante et al. (23), conducted a prospective, open-label study with 50 opioid-naïve cancer pain patients, of whom 39 completed the entire study. Patients were started on a 100 mg daily dose of TP ER. Throughout the study, pain intensity decreased significantly from baseline at all intervals (4 weeks; 1st week d = 1.85 and 4th week d = 3.17), while adverse effects did not change significantly. Patients who were successfully treated with TP ER had received a mean dose of less than 200 mg/day at the end of the study (after 4 weeks) and only 7 % of the patients discontinued TP ER because of side effects or low patient acceptance of the drug.
After one week of TP ER, our patients were successfully treated with less than 200 mg/day (77.36 %) and less than 100 mg/day (50.9 %), figures similar to the Mercadante et al. study. Our results were also similar with regard to the percentage of side effects (7.55 %) at one week of TP ER treatment. This rate can be considered acceptable.
In our series, patients treated with TP ER experienced significantly decreased pain when comparing VAS pre- and post-TP treatment values. We found a very strong analgesic effect in our cancer patients (d = 2.55), while as previously mentioned, our results for analgesic potency were similar to those of Mercadante et al. The most potent analgesic effects in our series may also be due to the added analgesic effects of radiation therapy.
This finding is in agreement with the analgesic response scale proposed by the International Bone Metastases Consensus Working Party (24), where a potent response was defined as pain reduction of four or more points in the VAS score with no concomitant increase in analgesic intake.
In addition, patients with higher pre-TP ER VAS scores did not need higher TP ER doses to obtain a good level of analgesia (X2 p < 0.05).
TP doses were maintained in 66.03 % of the patients, which supports the idea that TP ER has slow development of tolerance X2 (1) = 8.00. This is also similar to the results found by Mercadante et al.
Individual variation in sensitivity to different opioids may be the result of pharmacokinetic or pharmacodynamic effects associated with genetic polymorphisms that produce an asymmetric tolerance to the varied effects of different opioids in patients (32).
Opioid switching is a term given to the critical practice of substituting one opioid with another when a favorable balance between analgesia and adverse effects is not achieved with the first opioid (23). In our study 3 patients (5.66 %) were switched to TP ER because of side effects with sulfate of morphine (2) and oxycodone (1). Additionally, 4 patients were switched from TP ER to other drugs due to lack of good pain control. Results of this study are similar to those showed by Imanaka et al. (33) where only eight of 50 patients did not maintain pain control; two of those patients did not have pain intensity scores for 3 consecutive days.
It would be of interest to explore the conversion ratio between TP ER and other opioids, although it has been suggested that an equianalgesic ratio with oral morphine would be 1:2.5 (34).
The percentage of our patients who discontinued TP ER (11.32 %) seems to be even less than for patients treated with oral morphine (13 %), transdermal buprenorphine (15 %), or transdermal fentanyl (about 14 %), reported in previous studies with similar design and duration (20-22). In studies of non-cancer pain, TP ER was associated with a lower rate of discontinuation in comparison with oxycodone (7,14,15), mainly due to a better gastrointestinal tolerability profile since TP ER produce fewer opioid-related adverse effects that typical µ-opioid agonists (12).
Chronic pain has a high social cost, arising from direct costs (drugs, imaging, medical visits) and indirect costs (working days lost). In opioid-treated patients, management of side effects may represent a further cost, especially opioid induced constipation (OIC) as it persists for as long as opioid therapy is administered (35). Opioid use is costly to society and these costs vary with OIC severity (36). Cost effectiveness analyses are frequently used to guide health policy decisions, particularly in chronic pain management. However, head-to-head placebo-controlled trials are often not feasible: they are expensive and time-consuming. Adjusted indirect comparison of randomized controlled trials (RCTs) has become an increasingly accepted method for assessing the effect of pharmaceutical interventions (37,38).
The probability that tapentadol would be cost effective compared with each comparator at the willingness-to-pay threshold of #x20ac;20,000 to #x20ac;30,000/QALY gained exceeded 90 % in Spain (39). Similarly, Ikenberg et al. showed that TP ER can be used as second-line treatment instead of oxycodone ER in patients who failed first-line treatment with morphine, with a significant improvement in quality of life and with reduced costs for the NHS in the UK (40). Tapentadol ER was shown to provide better clinical outcomes at lower costs, by indirect comparison with oxicodone/naloxone ER. Therefore, TP ER is likely to be a cost effective first-line treatment in paitins with chronic, severe, musculoskeletal pain (41). These results should be validated in studies of TAP ER in tumor-related pain conditions.
A limitation of the present study is that opioid naïve patients will respond to any low dose opioids. Therefore, only taking into account at this patient group on TP could be not the best representation of cancer pain population of TP ER efficacy in particular, but we can find in the literature support to considered TP as a flexible drug to be use for the management of moderate-severe cancer pain (23). Likewise, side effect profiles of opioid are usually dose dependent. Hence, TP side effect profile could be not superior to other traditional opioids at low dose. But TP needs low escalation indexes than patients with other opioids and the percentage of patients who discontinued TP is less than patients treated with other opioids. Finally, there is no control group. Merely comparing before and after effect of a single agent is not scientific and can miss lead readers.
TP ER treatment resulted in an better global effect, reducing adverse effects while providing excellent analgesia (23).
Trials with a larger number of patients could identify subclasses of patients who could benefit from TP ER, which may be of particular benefit to opioid-naive patients or, for example, the elderly.
Conclusion
TP ER in doses of 100 mg/day was well-tolerated and effective in opioid-naive patients with cancer pain and could be considered as a flexible drug to be used for the management of moderate-to-severe cancer pain.
Patients were controlled with low doses and developed tolerance slowly.
TP ER has an analgesic role in the context of radiotherapy patients.
Controlled trials performed in cancer patients will identify subclasses of patients who could benefit from TP ER.
References
1. Gaertner J, Schiessl C. Cancer Pin Management: What's new? Curr Pain Headache Rep 2013;17:328. [ Links ]
2. National Cancer Institute. National Cancer Institute. www. 2012. Accessed Dec, 2012. [ Links ]
3. World Health Organiation (WHO). World Health Organization (WHO) www. 2012. Accessed Dec, 2012. [ Links ]
4. Shaheen PE, Legrand SB, Walsh D, Estfan B, Davis MP, Lagman RL, et al. Errors in opioid precribing: A prospective survey in cancer pain. J Pain Symptom Man 2010;39:702-11. Doi: 10.1016/j.jpainsymman.2009.09.009. [ Links ]
5. Goudas LC, Bloch R, Gialeli-Goudas M, Lau J, Carr DB. The epidemiology of cancer pain. Cancer Invest 2005;23:182-90. [ Links ]
6. Breuer B, Fleishman SB, cruciani RA, Portenoy RK. Medical oncologists' attitudes and practice in cancer pain management: A national survey. J Clin Oncol 2011;29:4769-75. Doi: 10.1200/JCO.2011.35.0561. [ Links ]
7. Afilalo M, Stegmann JU, Upmalis D. Tapentadol immediate release: A new treatment option for acute pain management. Journal of Pain Research 2010;3:1-9. [ Links ]
8. Crombie IK, Davies HT, Macrae WA. Cut and thrust: Antecedent surgery and trauma among patients attending a chronic pain clinic. Pain 1998;76(1-2):167-71. [ Links ]
9. Macrae WA. Chronic pain after surgery. Br J Anaesth 2001;87(1):88-98. [ Links ]
10. Taillefer MC, Carrier M, Belisle S, Levesque S, Lanctôt H, Boisvert AM, et al. Prevalence, characteristics, and predictors of chronic nonanginal postoperative pain after a cardiac operation: a cross-sectional study. J Thorac Cardiovasc Surg 2006;131(6):1274-80. [ Links ]
11. Mercadante S, Porzio G, Adile C, Aielli F, Cortegiani A, Dickenson A, et al. Tapentadol at Medium-High Doses in Patients previously receiving strong opioids for the management of cancer pain. Curr Med Res Opin 2014;30(10):2063-8. [ Links ]
12. Kress HG. Tapentadol and its two mechanisms of action: Is there a new pharmacological class of centrally-acting analgesics on the horizon? Eur J Pain 2010;14: 781-3. Doi: 10.1016/j.ejpain.2010.06.017. [ Links ]
13. Mercadante S, Ferrera P, Adile C. Switching from methadone to tapentadol for cancer pain. A case report. Journal of Pain and Symptom Management 2012 Sep;44(3):e3-5. doi: 10.1016/j.jpainsymman.2012.03.005. Epub 2012 Jul 21. [ Links ]
14. Buynak R, Shapiro DY, Okamoto A, Van Hove I, Rauschkolb C, Steup A, et al. Efficacy and safety of tapentadol extended release for the management of chronic low back pain: Results of a prospective, randomized, double-blind, placebo and active controlled phase III study. Expert Opin Pharmacother 2010;11:1787-804. Doi: 10.1517/14656566.2010.497720. [ Links ]
15. Wild J, Grond S, Kuperwasser B, Gilbert J, McCann B, Lange B, et al. Long-term safety and tolerability of tapentadol extended release for the management of chronic low back pain or osteoarthritis pain. Pain Prac 2010;10:416-27. [ Links ]
16. Falk S, Patel R, Heegaard A, Mercadante S, Dickenson AH. Spinal neuronal correlates of tapentadol analgesia in cancer pain: A back-translational approach. EJP 2014 Jun 11. doi: 10.1002/ejp.530 (Epub ahead of print). [ Links ]
17. Kress HG, Koch D, Kosturski H, Steup A, Karcher K, Lange B, et al. Tapentadol prolonged release for managing moderate to severe, chronic malignant tumor-related pain. Pain. Pain Phys 2014;17:329-43. [ Links ]
18. Imanaka K, Tominaga Y, Etropolski M, van Hove I, Ohsaka M, Wanibe M, et al. Efficacy and safety of oral tapentadol extended release in Japanese and Korean patients with moderate to severe, chronic malignant tumor-related pain. Curr Med Res Opin 2013;29(10):1399-409. [ Links ]
19. Portenoy RK. Clinical perspectives on preclinical models of bone pain: Questions and promises. Pain 2011;152:2455-6. [ Links ]
20. Mercadante S, Porzio G, Ferrera P, Fulfaro F, Aielli F, Ficorella C, et al. Low morphine doses in opioid-naive cancer patients with pain. J Pain Symptom Manage 2006;31:242-7. [ Links ]
21. Mercadante S, Porzio G, Ferrera P, Aielli F, Verna L, Tirelli W, et al. Low doses of transdermal buprenorphine in opioid-naive cancer patients with pain. Clin Ther 2009;25:1517-28. [ Links ]
22. Mercadente S, Porzio G, Ferrera P, Aielli F, Adile C, Ficorella C. Low doses of transdermal fentanyl in opioid-naive patients with cancer pain. Curr Med Res Opin 2010;26:2765-8. [ Links ]
23. Mercadante S, Porzio G, Ferrera P, Aielli F, Adile C, Ficorella C, et al. Tapentadol in cancer pin management: A prospective open-label study. Curr Med Res Opin 2012;28(11):1-5. [ Links ]
24. Chow E, Wu JS, Hoskin P, Coia LR, Bentzen SM, Blitzer PH. International consensus on palliative radiotherapy endpoints for future clinical trials in bone metastases. Radiotherapy Oncol 2002;64:275-80. [ Links ]
25. Cherny N, Ripamont C, Pereira J, Davis C, Fallon M, McQuay H, et al. Strategies to manage the adverse effects of oralmorphine: An evidence-based report. J Clin Oncol 2001;19:1887-93. [ Links ]
26. Caraceni A, Hanks G, Kaasa S, Bennett MI, Brunelli C, Cherny N, et al. Use of opioid analgesics in the treatament of cancer pain: Evidence-based recommendatios from the EAPC. Lancet Oncol 2012;13:e58-68. [ Links ]
27. Hoy SM. Tapentadol extended release: In adults with chronic pain. Drugs 2012;72:375-93. Doi: 10.2165/11208600-000000000-00000. [ Links ]
28. Afilalo M, Etropolski MS, Kuperwasser B, Kelly K, Okamoto A, Van Hove I, et al. Efficacy and safety of tapentadol extended release compared with oxycodone controlled release for the management of moderate to severe chronic pain related to osteoarthritis of the knee: A randomized, double-blind, palcebo and active controlled phase II study. Clin Drug Investig 2010;30:489-505. Doi: 10.2165/11533440-000000000-00000. [ Links ]
29. Schwartz S, Etropolsky M, Shapiro DY, Okamoto A, Lange R, Haeussler J, et al. Safety and eficay of tapentadol ER in patients with painful diabetic peripheral neuropathy: Results of a randomized-withdrawal, palcebo-controlled trial. Curr Med Res Opin 2011;27:151-62. Doi: 10.1185/03007995.2010.537589. [ Links ]
30. Schroder W, De Vry J, Tzschentke T, Jahnel U, Christoph T. Differential contribution of opioid and noradrenergic mechanisms of tapentadol in rats models of nociceptive and neuropathic pain. Eur J Pain 2010;14:814-21. [ Links ]
31. Shafer M. Novel concept for analgesia in severe pain- current and future implications. Eur J Pain Suppl 2009;3:6-10. [ Links ]
32. Mercadante S. Opioid rotation for cancer pain: Rationale and clinical aspects. Cancer 1999;86:1856-66. [ Links ]
33. Imanaka K, Tominaga Y, Etropolski M, Ohashi H, Hirose K, Matsumura T. Ready conversion of patients with well-controlled, moderate to severe, chronic malignant tumor-related pain on other opioids to tapentadol extended release. Clin Drugs 2014;34:501-11. [ Links ]
34. Tzschentke TM, De Vry J, Terliden R. Tapentadol hydrochloride: Analgesic mu-opioid receptor agonist noradrenaline reuptake inhibitor. Drugs Future 2006;31:1053-61. [ Links ]
35. Camilleri M. Opioid-induced constipation: Challenges and therapeutic opportunities. Am J Gastroenterol 2011;106:835-42. [ Links ]
36. Hjalte F, Berggren AC, bergendahl H, Hjortsberg C. The direct and indirect costs of opioid-induced constipation. J Pain symptom Mange 2010;40:696-703. [ Links ]
37. Song F, Loke YK, Walsh T, Glenny AM, Eastowood AJ, Altman DG. Methodological problems in the use of indirect comparisons for evaluating health care interventions: Survey of published systematic reviews. BMJ 2009;338:b1147. [ Links ]
38. Loannidis JP. Indirect comparisons: The mesh and mess of clinical trials. Lancet 2006;368:1470-2. [ Links ]
39. Obradovic M, Ikenberg R, Hertel N, Antoñanzas F, Gálvez R, Liedgens H. Cost-effectiveness of tapentadol in severe chronic pain in Spain: A cost analysis of data from RCTs. Clin Ther 2012;34:926-43. [ Links ]
40. Ikenberg R, Hertel N, Moore RA, Obradovic M, Baxter G, Conway P, et al. Cost-effectiveness of tapentadol prolonged release compared with oxycodone controlled release in the UK in patients with severe non-malignant chronic pain who failed 1st line treatment with morphine. J Med Econ 2012;15:724-36. [ Links ]
41. Coluzzi F, Ruggeri M. Clinical and economic evaluation of tapentadol extended release and oxycodene/naloxone extended release in comparison with controlled release oxycodone in musculoskeletal pain. Current Medical Research and Opinion 2014;30(6):1139-51. [ Links ]
![]() Correspondence:
Correspondence:
Escarlata López Ramírez
jaghgr@gmail.com
Recibido: 22-3-15.
Aceptado: 1-6-15.