My SciELO
Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista de la Sociedad Española del Dolor
Print version ISSN 1134-8046
Rev. Soc. Esp. Dolor vol.28 n.2 Madrid Mar./Apr. 2021 Epub June 21, 2021
https://dx.doi.org/10.20986/resed.2021.3825/2020
ORIGINALES
Postoperative ketamine efficacy in patients receiving chronic opioids undergoing spinal surgery
1Servicio de Anestesiología y Reanimación. Parc de Salut Mar. Barcelona, España
INTRODUCTION
Patients undergoing spine surgery experience moderate-severe pain during the postoperative period 1. VAS pain intensity records typically range from 5 to 8, depending on the procedure performed 1,2. At our center, 20 % of the patients who underwent surgery are receiving chronic opioid treatment before surgery. This incidence is similar to that described in the literature 3,4.
In the first 48 postoperative hours, patients on chronic opioid therapy have higher morphine requirements and VAS records 5. The use of non-opioid adjuvant drugs 6 is recommended to optimize its analgesic treatment. Low-dose ketamine is useful in this context 6,7.
In 2018, following the guidelines of APS 6, our center decided to change the postoperative analgesic protocol for patients on chronic opioid treatment scheduled for spine surgery. During the intraoperative period, the use of continuous ketamine infusion (CI) at low doses was protocolized. In the first 48 postoperative hours, the use of a PCA with morphine-ketamine was chosen to replace the protocol used up to that time, in which tramadol (100 mg/6 h) or a PCA with intravenous morphine was prescribed. In both protocols, multimodal analgesia with paracetamol and dexketoprofen prescribed is also considered.
The main objectives of this analysis were to compare pain intensity assessed using VNS and rescue opioid consumption in the first 48 postoperative hours between two cohorts of patients. One cohort consisting of patients who received either tramadol or morphine PCA (control group) and another cohort consisting of patients who received intraoperative ketamine with a PCA regimen with morphine-ketamine (ketamine group) in the first 48 postoperative hours.
MATERIAL AND METHODS
This study is a retrospective efficacy analysis of the new analgesic protocol implemented in the Anesthesiology Service of the Hospital del Mar in Barcelona compared with the previous protocol. No confidential data from the patient's medical records is published.
Inclusion and exclusion criteria
All patients treated with strong opioids or oral tramadol doses equal to or above 200 mg/24 h, for 6 weeks or more before any type of back surgery in 2017 and 2018 were included in the analysis.
Patients who did not successfully complete the analgesic protocol were excluded.
Analgesic protocol applied to the control group (the year 2017): Paracetamol 1 g/6 h i.v., dexketoprofen 50 mg/12 h i.v., tramadol 100 mg/6 h i.v. or morphine PCA 1 mg/ml (bolus 1 ml, closing time 10 min, maximum 6 bolus/h) according to the criteria of the responsible anesthesiologist. Rescue analgesia in the postoperative intensive care unit (POICU): Bolus of 2 mg morphine i.v. until a VNS < 3 is obtained as a first option, or continuous infusion of morphine at 1 ml/h is initiated as a second option. Rescue analgesia in the hospitalization unit: Bolus of subcutaneous morphine (s.c.)/4 h at doses of 0.01 to 0.05 mg/kg until a VNS < 3 is achieved.
Analgesic protocol applied to ketamine group (the year 2018): Ketamine bolus at a dose of 0.5 mg/ kg in anesthetic induction, followed by continuous infusion at a dose of 0.2 mg/kg/h until the closure of the surgical wound. In the postoperative period, paracetamol 1 g/6 h i.v., dexketoprofen 50 mg/8 h i.v., and PCA morphine-ketamine for 48 h were administered (preparation being 1 mg/1 mg, bolus 1 ml, closing time 10 min, maximum 6 bolus/h). Rescue analgesia at the POICU: Bolus of 2 mg morphine i.v. until a VNS < 3 is reached as the first option, or the initiation of continuous morphine-ketamine infusion at 1 ml/h as the second option. Rescue analgesia in the hospitalization unit: Morphine bolus s.c./4 h at doses of 0.01 to 0.05 mg/ kg until a VNS < 3 is achieved.
Assessed variables
All data were collected from the medical and nursing records of the patients' computerized medical records. The following demographic data were analyzed: Sex, age, and preoperative diagnosis. Regarding the preoperative analgesic treatment, the following data were collected: The type of opioid and the dose administered, the use of concomitant medication, and the performance of invasive analgesic techniques. The following data were collected on the surgical procedure: Performed surgical technique (laminectomy, arthrodesis, rearthrodesis, material removal, etc.), surgical time, level and spaces operated, and intraoperative opioid used. During the postoperative period, VNS records were collected at 2 h after the end of the surgery, and during 48 h postoperative period (evaluated every 8 h), the type of opioid prescribed, its dose, and the administration of rescue analgesia. Psychomimetic symptoms, impaired consciousness, respiratory depression, and nausea or vomiting were also reported.
Statistical analysis
The SPSS statistical package version 17 for Windows was used for statistical analysis. Mean and standard deviation were used in the description of continuous variables. The number and percentage of patients by response category were used for the description of categorical variables. Statistical techniques were used to ensure compliance with statistical assumptions before comparative testing. If the established assumptions were not met, the corresponding non-parametric tests were used. Comparative analyzes were performed only for categorical variables. The Chi-square test was applied in comparisons with the nominal dependent variable and the Mann-Whitney U test compared with the ordinal dependent variable. A statistical significance level of 0.05 was set for all statistical tests.
RESULTS
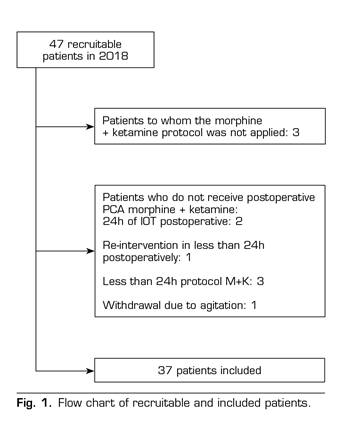
A total of 43 patients were included in the control group. In the ketamine group, 37 out of the 47 patients undergoing chronic opioid treatment in 2018 were included. Ten patients were excluded: Morphine-ketamine (M-K) protocol was not applied in 3 patients, and it was not fully applied in 7 patients (Figure 1).
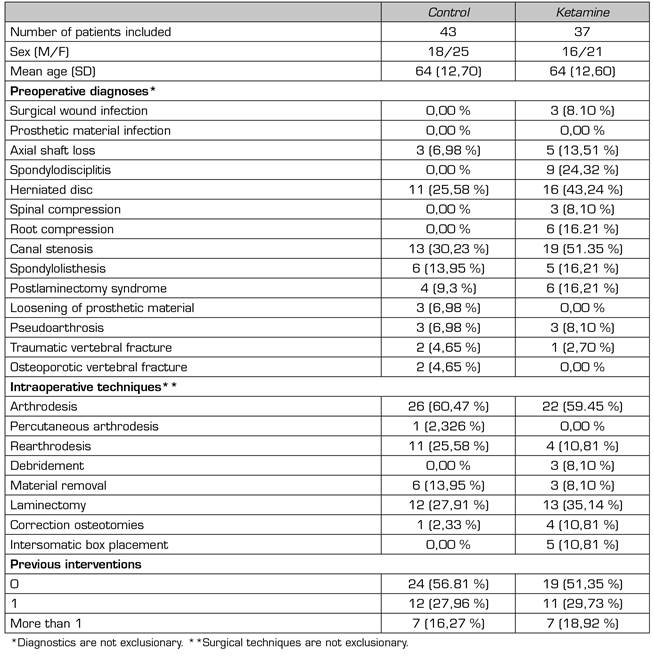
Demographic characteristics are similar in both groups (Table 1). The most frequent preoperative diagnoses were canal stenosis and disc hernia. The most frequent surgical technique was arthrodesis. A total of 50 % of patients underwent spinal surgery for the first time.
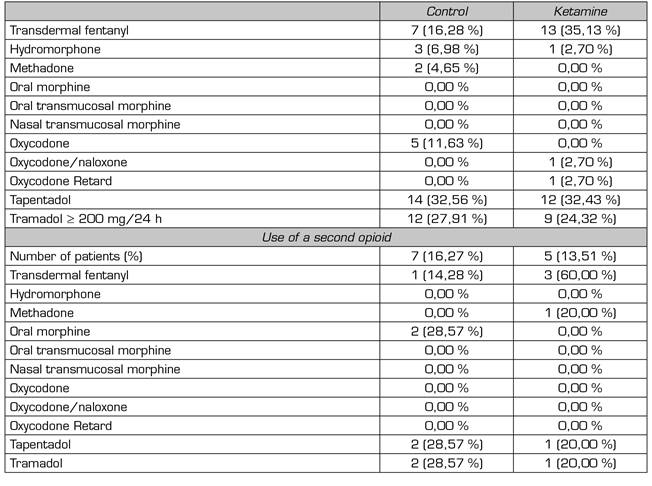
Tramadol, tapentadol, and transdermal fentanyl were the most preoperatively prescribed opioids in both groups. The percentage of patients receiving concomitant treatment with two opioid drugs was similar in both groups: 16.27 % control, 13.51 % ketamine (Table 2).
The mean preoperative morphine dose is expressed in oral milliequivalents (mEq) of morphine and was calculated according to the sources in Annex 1. In the control group, patients received a mean dose of 75.93 mEq, with a standard deviation (SD) of 59.97 mEq. In the ketamine group, the mean was 50 mEq (SD 71.60 mEq).
Annex I Table of opioid equivalence
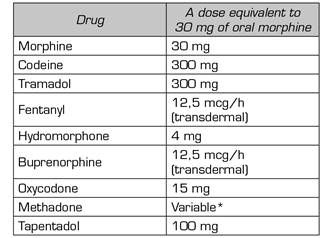
Source: O'Brien T, Christrup LL, Drewes AM, Fallon MT, Kress HG. European Pain Federation position paper on appropriate opioid use in chronic pain management. Eur J Pain. 2017;21(1):3-19. DOI: 10.1002/ejp.970.
*Use of 5:1 for conversion from methadone to oral morphine. (Source: Mercadante S, Casucio A, Calderone L. Rapid switching from morphine to methadone in cancer patients with poor response to morphine. J Clin Oncol. 1999;17[10]:3307-12. DOI: 10.1200/JCO.1999.17.10.3307.).
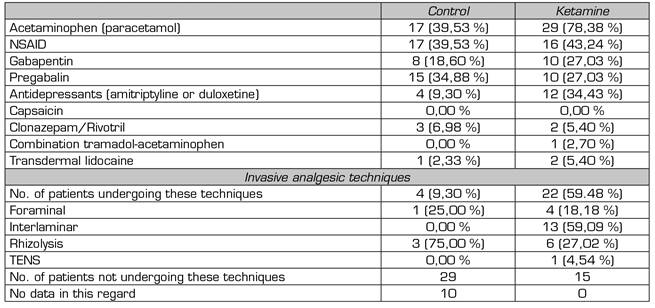
Preoperative, all patients analyzed performed multimodal analgesia combining at least one opioid with another analgesic drug (Table 3). In both groups, more than one-third received neuropathic pain medication.
The difference in the use of invasive analgesic techniques (control 8.5 % vs. ketamine 100 %) is not analyzable. For most patients in the control group (67 %), no data is available due to the absence of records in the electronic medical record.
During the intraoperative period, fentanyl was the most commonly used opioid (ketamine group 82.6 %, control group 93 %). The doses used ranged from 400 to 1100 mcg in both groups. The dosage varies depending on the intervention performed (e.g., discectomy vs. multilevel arthrodesis with osteotomies) and the required intraoperative opioid requirements according to the criteria of each anesthesiologist responsible for the surgery.
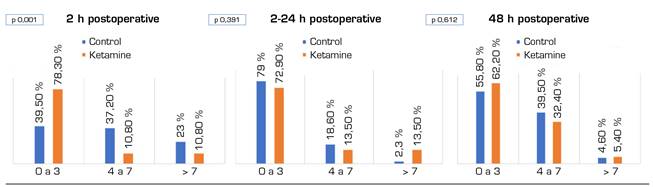
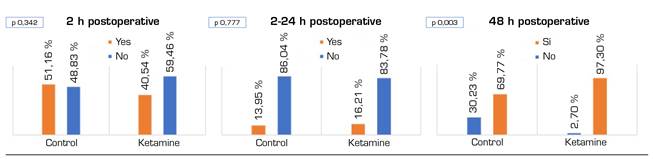
Figure 2 shows VNS records during the first two hours of the postoperative period, from 2 to 24 hours, and during the second day of the postoperative period. Except for the first 2 postoperative hours, during which the VNS of the ketamine group are decreased (p = 0.001), no differences between the two groups were found. Figure 3 shows the need for rescue morphine bolus over the same periods. There are statistically significant differences during the second postoperative day (p = 0.003) in favor of the ketamine group.
From the patient's electronic medical record, dose data could only be obtained for morphine bolus administered during the first 24 hours postoperatively. In the control group, a mean of 2.47 mg morphine i.v. (2-10 mg) was administered during the first 2 hours postoperatively. Between the first 2 and 24 hours, an average of 0.49 mg (0-10 mg) was administered. In the ketamine group, an average of 1.69 mg morphine i.v. (0-8 mg) was administered during the first 2 hours. Between 2 and 24 hours, a mean of 0.62 mg (0-8 mg) was administered.
Regarding the need for continuous infusion, either morphine in the control group or morphine-ketamine in the ketamine group, patients in the ketamine group had significantly lower needs (Figure 4).
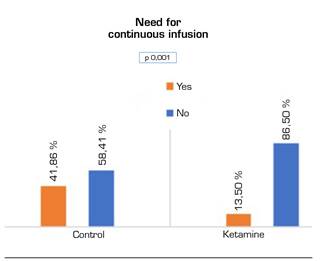
Fig. 4 Need for morphine infusion from admission to resuscitation to 48 postoperative hours. Chi-square test.
Concerning complications from analgesic treatment, none of the patients in the control group had psychomimetic symptoms, only 1 patient had postoperative nausea or vomiting (PONV), 6 patients had decreased level of consciousness (sleepiness), and 1 patient had bradypnea. In the ketamine group, 1 patient presented dysphoria and 3 patients presented PONV. None of the patients in the ketamine group had respiratory or level of consciousness changes.
DISCUSSION
With the implementation of the low-dose ketamine-based analgesia protocol in 2018, we have decreased the need for rescue opioids in our center and improved pain management in the first two postoperative hours. No higher incidence of adverse events has been recorded.
The number of patients with back pain on opioid therapy has increased in recent decades 8,9,10. Multimodal analgesia is the most correct approach, both pre-and postoperative 6,11. At subanesthetic doses, ketamine has been shown to decrease the requirements of postoperative opioids, especially in these types of patients 6,7,12,13.
The results found in our analysis are comparable to the results published by Loftus et al. 14 and Nielsen et al. 15. Both clinical trials demonstrated a decrease in postoperative opioid use and improved pain control in patients on chronic opioid therapy.
Similar to Nielsen et al., we decided to include patients treated with tramadol in the analysis. Although the minimum opioid dose at which a patient may have tolerance or secondary hyperalgesia is unknown, doses below 40 mg of oral morphine have been found to cause them 16.
A subanalysis was performed in the study conducted by Nielsen et al. to clarify whether patients with higher doses of morphine in the preoperative period had a higher decrease in morphic consumption in the postoperative period. It is noted that the reduction is higher with doses above 36 mEq oral morphine. In our study, no such analysis was performed because of the small number of patients included.
The use of high-dose opioids during the intraoperative period results in hyperalgesia and tolerance during the first two postoperative hours 17,18,19. However, continuous intraoperative ketamine infusion may prevent the hyperalgesia component by acting on NMDA receptors 20. In our center, intraoperative opioid needs may reach 1100 mcg of intravenous fentanyl. It has not been analyzed whether the main factor determining the need for such high doses is the largest surgical aggression or whether chronic opioid treatment is a relevant factor. In patients in the ketamine group, despite having intraoperative opioid needs similar to those in the control group, VNS records are significantly lower during the first two postoperative hours than in the control group. The effect of infusion at subanesthetic doses of ketamine during surgery may justify this finding.
In our center, patients are not moved in the first 24 postoperative hours. Pain management in this period is acceptable in both groups. However, sitting and early motion begins at 48 hours. In the case of the ketamine group, patients have better baseline analgesic control, requiring fewer rescue opioids. Maintaining ketamine in this period may be key because nociceptive stimulation is again of high intensity 12.
This analysis has some limitations. During the postoperative period, it was not possible to record the number of morphine PCA bolus or morphine + ketamine PCA bolus administered to each patient. Nor are the doses of rescue morphine administered in the hospital ward. We also do not know whether the VNS records are regarding moving or resting.
CONCLUSIONS
The use of ketamine during intraoperative and the first 48 postoperative hours in patients with chronic opioid treatment undergoing spinal surgery decreases the need for postoperative opioids and improves pain management in the first 2 postoperative hours. In the future, a prospective observational study would allow us to understand pain control fully, the need for rescue opioids, the degree of satisfaction, and the rate of complications in patients treated with ketamine. It would be interesting to know the effect of this drug after 6 and 12 months of the intervention because the use of intraoperative ketamine could decrease long-term pain and its chronification.
BIBLIOGRAFÍA
1. Polanco-García M, García-Lopez J, Fábregas N, Meissner W, Puig MM, Consortium POS. Postoperative Pain Management in Spanish Hospitals: A Cohort Study Using the PAIN-OUT Registry. J Pain. 2017;18(10):1237-52. DOI: 10.1016/j.jpain.2017.05.006. [ Links ]
2. Kim HJ, Park JH, Kim JW, Kang KT, Chang BS, Lee CK, et al. Prediction of postoperative pain intensity after lumbar spinal surgery using pain sensitivity and preoperative back pain severity. Pain Med. 2014;15(12):2037-45. DOI: 10.1111/pme.12578. [ Links ]
3. Jain N, Phillips FM, Weaver T, Khan SN. Preoperative Chronic Opioid Therapy: A Risk Factor for Complications, Readmission, Continued Opioid Use and Increased Costs After One- and Two-Level Posterior Lumbar Fusion. Spine (Phila Pa 1976). 2018;43(19):1331-8. DOI: 10.1097/BRS.0000000000002609. [ Links ]
4. Walid MS, Hyer L, Ajjan M, Barth AC, Robinson JS. Prevalence of opioid dependence in spine surgery patients and correlation with length of stay. J Opioid Manag. 2007;3(3):127-8, 30-2. [ Links ]
5. Patanwala AE, Jarzyna DL, Miller MD, Erstad BL. Comparison of opioid requirements and analgesic response in opioid-tolerant versus opioid-naÔve patients after total knee arthroplasty. Pharmacotherapy. 2008;28(12):1453-60. DOI: 10.1592/phco.28.12.1453. [ Links ]
6. Chou R, Gordon DB, de Leon-Casasola OA, Rosenberg JM, Bickler S, Brennan T, et al. Management of Postoperative Pain: A Clinical Practice Guideline From the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists' Committee on Regional Anesthesia, Executive Committee, and Administrative Council. J Pain. 2016;17(2):131-57. DOI: 10.1016/j.jpain.2015.12.008. [ Links ]
7. Schwenk ES, Viscusi ER, Buvanendran A, Hurley RW, Wasan AD, Narouze S, et al. Consensus Guidelines on the Use of Intravenous Ketamine Infusions for Acute Pain Management From the American Society of Regional Anesthesia and Pain Medicine, the American Academy of Pain Medicine, and the American Society of Anesthesiologists. Reg Anesth Pain Med. 2018;43(5):456-66. DOI: 10.1097/AAP.0000000000000806. [ Links ]
8. Maher C, Underwood M, Buchbinder R. Non-specific low back pain. Lancet. 2017;389(10070):736-47. DOI: 10.1016/S0140-6736(16)30970-9. [ Links ]
9. Carames M, Robaina F, Calvo B. Opioides en el dolor raquídeo. Relación riesgo/beneficio y estrategia apropiada para su utilización. Rev Soc Esp Dolor. 2010;17(3):169-76. DOI: 10.1016/S1134-8046(10)70027-6. [ Links ]
10. Deyo RA, Von Korff M, Duhrkoop D. Opioids for low back pain. BMJ. 2015;350:g6380. DOI: 10.1136/bmj.g6380. [ Links ]
11. Mugabure Bujedo B, Gonzalez Santos S, Tranque Bizueta I, Araujo Lopez A, Toran Garcia L. Manejo del dolor perioperatorio de los pacientes en tratamiento crónico con opioides. Rev Soc Esp Dolor. 2009;16(5):288-97. DOI: 10.1016/S1134-8046(09)72038-5. [ Links ]
12. Himmelseher S, Durieux ME. Ketamine for perioperative pain management. Anesthesiology. 2005;102(1):211-20. DOI: 10.1097/00000542-200501000-00030. [ Links ]
13. Garg N, Panda NB, Gandhi KA, Bhagat H, Batra YK, Grover VK, et al. Comparison of Small Dose Ketamine and Dexmedetomidine Infusion for Postoperative Analgesia in Spine Surgery--A Prospective Randomized Double-blind Placebo Controlled Study. J Neurosurg Anesthesiol. 2016;28(1):27-31. DOI: 10.1097/ANA.0000000000000193. [ Links ]
14. Loftus RW, Yeager MP, Clark JA, Brown JR, Abdu WA, Sengupta DK, et al. Intraoperative ketamine reduces perioperative opiate consumption in opiate-dependent patients with chronic back pain undergoing back surgery. Anesthesiology. 2010;113(3):639-46. DOI: 10.1097/ALN.0b013e3181e90914. [ Links ]
15. Nielsen RV, Fomsgaard JS, Siegel H, Martusevicius R, Nikolajsen L, Dahl JB, et al. Intraoperative ketamine reduces immediate postoperative opioid consumption after spinal fusion surgery in chronic pain patients with opioid dependency: a randomized, blinded trial. Pain. 2017;158(3):463-70. DOI: 10.1097/j.pain.0000000000000782. [ Links ]
16. Carroll IR, Angst MS, Clark JD. Management of perioperative pain in patients chronically consuming opioids. Reg Anesth Pain Med. 2004;29(6):576-91. DOI: 10.1016/j.rapm.2004.06.009. [ Links ]
17. Kim D, Lim HS, Kim MJ, Jeong W, Ko S. High-dose intraoperative remifentanil infusion increases early postoperative analgesic consumption: a prospective, randomized, double-blind controlled study. J Anesth. 2018;32(6):886-9. DOI: 10.1007/s00540-018-2569-6. [ Links ]
18. Chia YY, Liu K, Wang JJ, Kuo MC, Ho ST. Intraoperative high dose fentanyl induces postoperative fentanyl tolerance. Can J Anaesth. 1999;46(9):872-7. DOI: 10.1007/BF03012978. [ Links ]
19. Hansen EG, Duedahl TH, Rmsing J, Hilsted KL, Dahl JB. Intra-operative remifentanil might influence pain levels in the immediate post-operative period after major abdominal surgery. Acta Anaesthesiol Scand. 2005;49(10):1464-70. DOI: 10.1111/j.1399-6576.2005.00861.x. [ Links ]
20. Peltoniemi MA, Hagelberg NM, Olkkola KT, Saari TI. Ketamine: A Review of Clinical Pharmacokinetics and Pharmacodynamics in Anesthesia and Pain Therapy. Clin Pharmacokinet. 2016;55(9):1059-77. DOI: 10.1007/s40262-016-0383-6. [ Links ]
Received: June 16, 2020; Accepted: March 22, 2021











 text in
text in