My SciELO
Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Enfermería Global
On-line version ISSN 1695-6141
Enferm. glob. vol.19 n.58 Murcia Apr. 2020 Epub May 18, 2020
https://dx.doi.org/eglobal.389551
Originals
Failure mode and effect analysis in the preparation and dispensation of chemotherapy
1Departament of Public Health. Federal University of Rio Grande do Norte. Natal/RN, Brazil.
Aim
Conduct a Failure Mode and Effect Analysis (FMEA) to prospectively identify the risks related to the preparation and dispensation of chemotherapy drugs at an outpatient unit of a reference center in oncology.
Methods
The first six stages of Failure Mode and Effect Analysis were used to identify dangerous situations and assemble a team; define the process to be analyzed and describe it graphically; apply a host of ideas to identify failure modes; prioritize failure modes and conduct risk analysis; identify potential causes of failure modes and redesign the process.
Results
Seventeen failure modes were identified, two of which were classified as high risk: changing the output window for the drug and miscalculating the intrathecal drug dose.
Conclusions
The possible failure modes related to the process analyzed were identified; in addition, it was possible to define potential causes of these risks.
Keywords: Healthcare Failure Mode and Effect Analysis; Patient Safety; Medication Errors
INTRODUCTION
In recent years, health services have been increasingly concerned with medical errors. A number of initiatives related to patient safety have emerged, including the landmark 1999 report entitled “To err is human”: building a safer health system” by the Institute of Medicine (IOM) of the United States. According to the IOM, errors associated with health care cause between 44,000 and 98,000 deaths a year in American hospitals1. This report revealed the number of errors and adverse events (AE) that occur in health services, harming patients, lengthening hospital stays, increasing social costs and even causing premature death2.
According to the World Health Organization (WHO), safety means “reduction, to an accepTable limit, in the risk of unnecessary harm associated with health care”3. Safe drug use aims at reducing medication errors, which is divided into several stages with high risk of failure.
Medication error is defined as an avoidable event that leads or may potentially lead to the inadequate use of medication. Errors may be related to professional practice, the products used, procedures, communication problems, including prescription, labels, packaging, names, preparation and dispensation (the focus of the present study), distribution, administration, education, follow-up and medication use4. Despite the potential seriousness of these events, it is believed that many are under-reported5.
Antineoplastic drugs are considered potentially dangerous and errors derived from their use may cause permanent injury or even death7. Added to this is the fact that these drugs exhibit a low therapeutic index, that is, the toxic dose is very near the effective dose, representing a high risk of adverse events. Chemotherapy involves the simultaneous use of several adjuvant drugs aimed at producing a synergistic therapeutic effect with the least toxicity possible7.
Studies demonstrate that errors related to antineoplastic drugs occur at a rate of 1 to 4 in every 1000 drug prescriptions, affecting from 1 to 3% of oncological patients, both children and adults, at all stages of the drug dispensation process8.
The literature highlights many errors in the prescription and administration 9 10 11, but there is little emphasis on this phase. The Institute for Safe Medication Practices (ISMP) recommends applying the risk management tool Failure Mode and Effects Analysis (FMEA) in order to minimize failures in the entire medication process12.
This tool is used by the industry to proactively assess potential failures in a certain process13 14. It has undergone several updates and is widely applied in different sectors such as the aerospace and automobile industries, among others. Healthcare Failure Mode and Effects Analysis (HFMEA) is a simplified form of Failure Mode and Effect Analysis FMEA) that has been adapted to health services. This makes it possible to proactively identify the vulnerabilities of health care systems. It is based on the concept that risk is not only related to the probability that failure will occur, but also the severity of its consequences13 14. After identification and analysis of failure modes, their effects and causes, intervention priorities and recommendations are established in order to identify the changes needed to eliminate or reduce the risks of possible failures. The process is analyzed from start to finish by a group of specialists in the area assessed. There are currently few studies on FMEA or HFMEA in Brazil, particularly the analysis and prevention of risks associated with chemotherapy drugs, demonstrating a clear need to apply FMEA as prospective assessment of failure modes and their effects in the dispensation and preparation of chemotherapy drugs, corroborating towards better patient quality and safety.
Thus, the present study aimed at conducting a multimode analysis of failures and effects to prospectively identify the risks related to the preparation and dispensation of chemotherapy drugs in an outpatient unit of an oncology reference center.
MATERIALS AND METHODS
This is a descriptive study that applied failure mode and effect analysis (FMEA), conducted in an outpatient unit of a philanthropic oncology reference center, in Natal, Rio Grande do Norte state, Brazil. FMEA consists of eight stages: 1- identifying dangerous situations and assembling a team; 2- defining the process to be analyzed and describing it graphically; 3- applying a host of ideas in order to identify failure modes; 4- prioritizing failure modes and conducting risk analysis; 5- identifying potential causes of failure modes; 6- redesigning the process; 7- analyzing and testing the new process; and 8- devising interventions and indicators 15. In the present study, we report only on stages 1 to 6, which were feasible to analyze at the facility in question.
Stage 1: identifying dangerous situations and assembling a team
FMEA was applied in the chemotherapy drug preparation and dispensation phase by the pharmacy service of a unit where outpatient chemotherapy sessions are held. The pharmacy service of the aforementioned unit produces an average of 3000 parenteral chemotherapy drug preparations per month or approximately 125 per day, 1700 hormone therapies, and oral chemotherapy.
The process to be evaluated was selected because of its potentially high risk or vulnerability16 17. Given the large volume of chemotherapy drugs handled (125 preparations/day) and administered in the reference hospital, medical error was proposed for the application of FMEA.
The tool was applied by a multidisciplinary team, consisting of professionals involved in the preparation and dispensation of chemotherapy drugs. The following criteria were established to select the team: having at least 2 years' experience in the chemotherapy area, accepting to participate in the FMEA development process, and being available to attend the meetings.
The team was composed of four pharmacists with three to twelve years' experience in the area, two nursing specialists with experience between five and twenty-three years, one nursing technician with four years' experience and a researcher with twelve years' experience in oncology nursing as mediator. The team received an explanatory pamphlet describing all the FMEA stages and their objectives. All the team meetings were held during the participants' working hours.
Stage 2: defining the process to be analyzed and describing it graphically
The medication process starts with a medical assessment of the patient, as well as the results of laboratory examinations to then evaluate the possible prescription or not of chemotherapy drugs. Next, the doctor enters a digital prescription into the computer and attaches a copy to the patient's medical chart. Patients are then referred to the chemotherapy administration sector, where they are registered, their dose noted and a drug label printed. Within this process, the subprocess medication “preparation and dispensation” was selected.
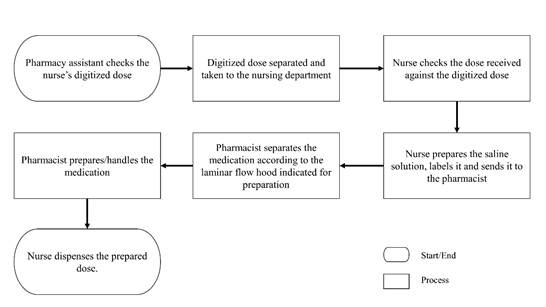
The flowchart of the medication preparation subprocess was created at the first meeting based on the knowledge of the team involved. This knowledge was essential, since it made it easy to identify the failure modes in each of the preparation and dispensation stages, involving pharmacy and nursing professionals (Figure 1).
Chemotherapy preparation and dispensation subprocess, Pharmacy assistant checks the nurse's digitized dose, Digitized dose separated and taken to the nursing department, Nurse checks the dose received against the digitized dose, Nurse prepares the saline solution, labels it and sends it to the pharmacist, Pharmacist separates the medication according to the laminar flow hood indicated for preparation, Pharmacist prepares/handles the medication, Nurse dispenses the prepared dose.
Stage 3: applying a host of ideas to identify failure modes
Four 90-minute meetings were held with the entire team. In the first meeting the FMEA tool was explained, as well as the instruments used in its application. Rules were established for cases of divergent results and consensus determined decision-making. After the process to be analyzed was defined and the subprocess selected, possible failure modes were identified for each of the stages using the brainstorming method.
Stage 4: prioritizing failure modes and analyzing risks
The failure modes were grouped into a Table and the Risk Assessment Matrix was applied to determine the risk severity versus probability of occurrence.
The Risk Assessment Matrix result is obtained by multiplying the probability of failure (frequent, occasional, uncommon, remote) by the severity of its consequences (catastrophic, important, moderate, minor). Failure modes with values of eight or higher are failures with risk of occurrence where the potential causes should be identified. In order to decrease the subjectivity of each criterion in terms of the severity of the effect and probability of occurrence, the classification proposed by the Veteran Affairs National Center for Patient Safety (VA) was used (Table 1)16.
Table 1: Criteria to classify the severity of failures analyzed with FMEA

Source: DeRosier J, et al. 16 and adapted by the authors.
For failure modes with scores ≥ 8, the decision tree was applied for HFMEA (Figure 2), which considers two additional criteria: criticality (if the failure is essential to the process) and possibility of detection (if its emergence is obvious and can be changed in time)16. After the decision tree was applied, it was possible to determine which failure modes should proceed to the next stage. Based on the results obtained, a proposal of measures to improve risk prevention was sent to the technician in charge of the pharmacy.
Ethical aspects
This study, which aimed at an in-depth investigation of situations that emerge in professional practice and do not reveal data that identify participants, complies with article VII guidelines of National Health Council of Brazil Resolution no. 510/2016, related to the protocols of the participating institution. The objective is to contribute to patient safety in the stages of chemotherapy medication preparation and dispensation.
RESULTS
A total of 17 failure modes were identified in the medication preparation and dispensation phase (Table 2).
Of the 17 failure modes, three obtained a value > 8 and were therefore analyzed with the decision tree for HFMEA: changing the output window of prepared medication, wrong calculation of intrathecal drug dose and wrong volume of aspirated medication. The failure mode “wrong drug volume aspirated during preparation” was interrupted by the decision tree, since it can be detected as it occurs. Thus, there are two potential failure modes that proceeded to the next FMEA stage, in order to propose improved measures and practices.
Stage 5: identifying the potential causes of failure modes
Several potential causes were identified by the team, showing possible failure hitherto not perceived, as described in Table 3.
With respect to the potential causes of failure modes related to “changing the output window of prepared medication” the possible causes are lack of attention, window labeling and knowledge of processes on the part of professionals. This failure could cause new drug administration in a prepared saline solution and an overdose, putting patients at risk, depending on the drug in question.
The failure mode “wrong calculation of intrathecal drug dose” could seriously harm the patient, and sometimes be fatal 18. The potential causes of this failure are a defective calculator, a change in drug presentation or work overload.
Stage 6: process redesign
Based on the identification of possible failure modes related to “changing the output window for the drug to an input window”, the process was redesigned as follows: in relation to “lack of attention”, a limit in the number of drugs handled at one time was stipulated using a diary of number of drugs handled per hour. In regard to “lack of window labeling”, windows will be labeled (both internally and externally) through working instructions. With respect to “lack of process knowledge”, systematic training will be provided based on existing protocols in the sector.
In terms of “wrong calculation of the intrathecal drug dose”, the following changes were suggested: for the cause “defective calculator”, it was recommended that drug doses be calculated with different calculators. For “change in drug presentation”, it was suggested that the institution itself print labels, and for "work overload", that the drugs to be dispensed be double checked.
DISCUSSION
Applying the FMEA tool at the pharmacy unit made it possible to identify possible failure modes that were hitherto undetected, demonstrating potential vulnerabilities in the chemotherapy drug preparation and dispensation process.
According to the comprehensive review, the main risk factors identified for the occurrence of errors related to drug preparation are associated with psychological factors, such as work overload, factors related to the work environment and outdated health education19.
The excess workload o
f health professionals is one of the risk factors most cited as a trigger of stress and lack of attention, facilitating errors20 21. Other studies also report overwork as a cause of failures 22 23. Given the high number of drugs handled and the fact that many professionals work double shifts, it is recommended that the pharmacist be limited in the number of drugs handled at one time.
With respect to workplace-related failures, Brazilian guidelines stipulate that the medication preparation area must be well lit and ventilated, and that access be restricted to professionals directly involved in the process to minimize distractions24. The physical infrastructure of the pharmacy where the study was held is adequate, well lit and equipped with physical barriers between the sectors, but there is a lack of labeling regarding the processes that are performed in each area.
Continuing education aims to share knowledge of the existing protocols in the sector with a maximum number of individuals involved in the chemotherapy medication process, thereby ensuring the quality of professional training at the institution. Health education is one of the tools that seeks to incorporate the team in a participative and dynamic way through classes, conversations or knowledge sharing. Among the limitations identified is the difficulty in gathering the entire team for all the scheduled meetings, due to their limited available time. The number of meetings may have restricted the possibility of conducting an in-depth investigation of other non-identified failure modes. Another difficulty is the consensus of the participants about the seriousness of the failures identified, where opinions varied considerably from one individual to another. This may be due to the length of experience of the workers or the circumstances of different occupations.
One of the FMEA limitations is the tendency to over or underestimate the seriousness of the failure, given it is a prospective possibility25. This tendency was observed in our application of FMEA, where the failure modes scored far below the “expected” value when decided by consensus.
Another study limitation is the fact that it was conducted in a single pharmacy, specialized in chemotherapy, with specific local characteristics. As such, the results obtained may not be common to other pharmacies specialized in chemotherapy. The present study proposed to apply FMEA to identify prospective failure modes and suggest interventions. A future study will make it possible to assess the effectiveness of measures taken in relation to stages 7 and 8.
During this study, prospective analysis of the process allowed ample assessment of possible failure modes. The study demonstrated the need for risk management in institutions that prioritize quality care, a concern that is only now emerging in our context.
Thus, with the increasing search for quality and patient safety, FMEA should be considered a valid method for enhancing risk management, since it allows a prospective analysis of the chemotherapy medication process in the preparation and dispensation phase, with a view to identifying potential failures and their associated causes, in addition to devising strategies to correct these vulnerabilities. Moreover, the involvement of employees in the patient safety process encourages a culture of institutional safety and more adequate management of the work process, thereby improving the quality of care provided to the users of health services.
CONCLUSION
The present study aimed at conducting a multimode analysis of failures and effects to prospectively identify the risks related to the preparation and dispensation of chemotherapy drugs in an outpatient unit of an oncology reference center. Within the proposed context, the possible failure modes related to chemotherapy preparation and dispensation were identified, in addition to establishing the potential causes of these risks.
With respect to the local context, this study was important in identifying possible opportunities to improve the identification, preparation and dispensation of chemotherapy medication, via application of the FMEA instrument. These contributions will minimize the current risks to patients, in addition to providing the employees involved in the medication process with knowledge of possible failures and control strategies aimed at containing risks.
REFERENCIAS
1. National Patient Safety Foundation. Free From Harm: Accelerating Patient Safety Improvement Fifteen Years After To Err Is Human. Boston: National Patient Safety Foundation; 2015 Available from: http://www.ihi.org/resources/Pages/Publications/Free-from-Harm-Accelerating-Patient-Safety-Improvement.aspx [ Links ]
2. Sousa P, Uva AS, Serranheira F, Nunes C, Leite ES. Estimating the incidence of adverse events in Portuguese hospitals: a contribution to improving quality and patient safety. BMC Health Serv Res [serial on the Internet]. 2014 [access: 22 out 2018];14(1):311. Available from: https://doi.org/10.1186/1472-6963-14-311 [ Links ]
3. World Health Organization. Marco Conceptual de la Classificación Internacional para la Seguridad del Paciente, version 1.1. Geneva: WHO/IER/PSP; 2009 [access: 05 mar 2018]. Available from: http://www.who.int/patientsafety/implementation/icps/icps_full_report_es.pdf [ Links ]
4. Santos J da SD, Almeida PHRF, Rosa MB, Perini E, Pádua CAM de, Lemos G da S. Prescription and Administration Errors Involving a Potentially Dangerous Medicine. J Nurs UFPE line [periódico na Internet]. 2017 [acesso: 05 mar 2018];11(10). Disponível em: https://doi.org/10.5205/1981-8963-v11i10a13807p3707-3717-2017 [ Links ]
5. Mendes W, Pavão ALB, Martins M, Moura MLO, Travassos C. The feature of prevenTable adverse events in hospitals in the State of Rio de Janeiro, Brazil. Rev Assoc Med Bras [periódico na Internet]. 2013 [acesso: 05 mar 2018];59(5). Disponível em: https://doi.org/10.1016/j.ramb.2013.03.002 [ Links ]
6. Costa NN, de Camargo Silva AEB, de Lima JC, de Sousa MR, Barbosa JSDF, Bezerra ALQ. O retrato dos eventos adversos em uma clínica médica: análise de uma década. Cogitare enferm [periódico na Internet]. 2016 [acesso: 22 out 2018];21(5): 01-10. Disponível em: http://dx.doi.org/10.5380/ce.v21i5.45661 [ Links ]
7. Mattsson TO, Holm B, Michelsen H, Knudsen JL, Brixen K, Herrstedt J. Non-intercepted dose errors in prescribing anti-neoplastic treatment: a prospective, comparative cohort study. Ann Oncol [serial on the Internet]. 2015 [access: 22 out 2018];26(5):981-6. Available from: https://doi.org/10.1093/annonc/mdv032 [ Links ]
8. Weingart SN, Zhang L, Sweeney M, Hassett M. Chemotherapy medication errors. Lancet Oncol [serial on the Internet]. 2018 [access: 22 out 2018 ];19(4):e191-9. Available from: https://doi.org/10.1016/S1470-2045(18)30094-9 [ Links ]
9. Raban MZ, Westbrook JI. Are interventions to reduce interruptions and errors during medication administration effective?: A systematic review [serial on the Internet]. BMJ Qual Saf. 2014 [access : 05 mar 2018]. 23. Available from: https://doi.org/10.1136/bmjqs-2013-002118 [ Links ]
10. Michaelson M, Walsh E, Bradley CP, McCague P, Owens R, Sahm LJ. Prescribing error at hospital discharge: a retrospective review of medication information in an Irish hospital. Ir J Med Sci [serial on the Internet]. 2017 [access: 05 mar 2018]; 186(3): [about 6 p.]. Available from: https://doi.org/10.1007/s11845-017-1556-5 [ Links ]
11. Keers RN, Williams SD, Cooke J, Ashcroft DM. Prevalence and Nature of Medication Administration Errors in Health Care Settings: A Systematic Review of Direct Observational Evidence. Ann Pharmacother [serial on the Internet]. 2013 [access: 22 out 2018];47(2):237-56. Available from: https://doi.org/10.1345/aph.1R147 [ Links ]
12. Instituto para Práticas Seguras no Uso de Medicamentos. Antineoplásicos parenterais: erros de medicação, riscos e práticas seguras na utilização. Belo Horizonte: ISMP; 2014 [acesso: 05 mar 2018]. Disponível em: http://www.ismp-brasil.org/site/wp-content/uploads/2015/07/V3N3.pdf [ Links ]
13. Polancich S, Rue L, Poe T, Miltner R. Proactive Risk Mitigation: Using Failure Modes and Effects Analysis for Evaluating Vascular Access. J Healthc Qual [serial on the Internet]. 2018 [access: 05 mar 2018]; 40(1). Available from: https://doi.org/10.1097/JHQ.0000000000000125 [ Links ]
14. Jain K. Use of failure mode effect analysis (FMEA) to improve medication management process. Int J Health Care Qual Assur [serial on the Internet]. 2017 [access: 5 mar 2018];30(2). Available from: https://doi.org/10.1108/IJHCQA-09-2015-0113 [ Links ]
15. Joint Commission Resources Inc. Failure Mode and Effects Analysis in Health Care: proactive risk reduction. Oakbrook Terrace (IL): Joint Commission Resources; 2010. [ Links ]
16. DeRosier J, Stalhandske E, Bagian JP, Nudell T. Using Health Care Failure Mode and Effect AnalysisTM: The VA National Center for Patient Safety's Prospective Risk Analysis System. Jt Comm J Qual Improv [serial on the Internet]. 2002 [access: 05 mar 2018];28(5). Available from: https://doi.org/10.1016/S1070-3241(02)28025-6 [ Links ]
17. Li G, Xu B, He R., Zhang S. Using Healthcare Failure Mode and Effect Analysis to Reduce Intravenous Chemotherapy Errors in Chinese Hospitalized Patients. Cancer Nurs [serial on the Internet]. 2017 [access: 22 out 2018]; 40(2):88-93. Available from: http://dx.doi.org/10.1097/ncc.0000000000000348 [ Links ]
18. Gilbar PJ. Intrathecal chemotherapy: potential for medication error. Cancer Nurs [serial on the Internet]. 2014 [access: 22 out 2018];37(4):299-309. Available from: http://dx.doi.org/10.1097/NCC.0000000000000108 [ Links ]
19. Camerini FG, Colcher AP, Moraes DS, Souza DL, Vasconcelos JR, Neves RO. Fatores de risco para ocorrência de erro no preparo de medicamentos endovenosos: uma revisão integrativa. Cogitare Enferm [períodico na Internet]. 2014 [acesso: 05 mar 2018]; 19(2). Disponível em: http://dx.doi.org/10.5380/ce.v19i2.37362 [ Links ]
20. Forte ECN, Machado FL, Pires DEP. A relação da enfermagem com os erros de medicação: uma revisão integrativa. Cogitare Enferm [períodico na Internet]. 2016 [acesso: 05 mar 2018]; 21. Disponível em: http://dx.doi.org/10.5380/ce.v21i5.43324 [ Links ]
21. Silva BA, Marques IB, Brasil POR, Cardoso AFRC, Pinto MNFB, Souza MMT. O trabalho da enfermagem no âmbito do SUS - estudo reflexivo. R Flu Exten Univ [periódico na Internet] 2017 [acesso: 05 mar 2018]; 07(1). Disponível em: http://editorauss.uss.br/index.php/RFEU/article/view/914/pdf [ Links ]
22. Teixeira TCA, De Bortoli CSH. Análise de causa raiz de acidentes por quedas e erros de medicação em hospital. ACTA Paul Enferm [periódico na Internet]. 2014 [acesso: 05 mar 2018]; 27(2). Disponível em: http://dx.doi.org/10.1590/1982-0194201400019 [ Links ]
23. Marini DC, Pinheiro JT, Integradas F, Imaculada M, Paula R. Avaliação dos erros de diluição de medicamentos de administração intravenosa em ambiente hospitalar para o desenvolvimento de um guia de diluição e administração dos mesmos. Infarma - Ciências Farm [periódico na Internet]. 2016 [acesso: 05 mar 2018];28(2). Disponível em: http://dx.doi.org/10.14450/2318-9312.v28.e2.a2016.pp81-89 [ Links ]
24. Brasil. Agência Nacional de Vigilância Sanitária. Resolução RDC N.º 45, de 12 de março de 2003. Dispõe sobre o Regulamento Técnico de Boas Práticas de Utilização das Soluções Parenterais (SP) em Serviços de Saúde. Brasília: ANVISA; 2003 [acesso: 05 mar 2018]. Disponível em: https://www20.anvisa.gov.br/segurancadopaciente/index.php/legislacao/item/resolucao-rdc-n-45-de-12-de-marco-de-2003 [ Links ]
25. McElroy LM, Khorzad R, Nannicelli AP, Brown AR, Ladner DP, Holl JL. Failure mode and effects analysis: A comparison of two common risk prioritisation methods. 2016 [access: 22 out 2018] BMJ Quality & Safety;25(5):329-36. Available from: http://dx.doi.org/10.1136/bmjqs-2015-004130 [ Links ]
Received: July 13, 2019; Accepted: October 17, 2019











 text in
text in