My SciELO
Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Medicina Oral, Patología Oral y Cirugía Bucal (Ed. impresa)
Print version ISSN 1698-4447
Med. oral patol. oral cir. bucal (Ed.impr.) vol.9 n.4 Aug./Oct. 2004
Applications of exfoliative cytology in the diagnosis of oral cancer
DINIZ-FREITAS M, GARCÍA-GARCÍA A, CRESPO-ABELLEIRA A, MARTINS-CARNEIRO JL, GÁNDARA-REY JM. APPLICATIONS OF EXFO-LIATIVE CYTOLOGY IN THE DIAGNOSIS OF ORAL CANCER. MED ORAL 2004;9:355-61.
SUMMARY
Exfoliative cytology is a simple non-aggressive technique that is well accepted by the patient, and that is therefore an attractive option for the early diagnosis of oral cancer, including epithelial atypias and especially squamous cell carcinoma. However, traditional exfoliative cytology methods show low sensitivity (i.e. a high proportion of false negatives) in the diagnosis of these pathologies. This low sensitivity is attributable to various factors, including inadequate sampling, procedural errors, and the need for subjective interpretation of the findings. More recently, the continuing development of automated cytomorpho-metric methods, DNA content determination, tumour marker detection, and diverse molecular-level analyses has contributed to renewed interest in exfoliative cytology procedures for the diagnosis of oral cancer. The present study briefly reviews developments in these areas.
Key words: Oral cancer, exfoliative cytology, cytomorphology, DNA analysis, tumour markers, molecular analysis.
INTRODUCTION
-Oral cancer
Over 90% of cancers of the oral cavity are squamous cell carcinomas (SCCs) (1). In Spain, the incidence of oral cancer is 12 - 15 cases per 100,000 per annum in men, and 2 - 4 cases per 100,000 per annum in women; oral cancers account for 2 - 3% of all cancer deaths (2). The 5-year survival rate is 80% for the initial stages, 40% for neoplasms with regional involvement, and less than 20% after metastasis (3). Early detection of asymptomatic early stages offers not only an increased probability of survival, but also improved quality of life in view of the reduced need for aggressive or disfiguring treatments (4). However, studies in the US (5) indicate that at the moment of diagnosis of oral cancer, about 36% of patients show localized disease, 43% regional involvement, and 9% metastasis; in the remaining 12% stage cannot be identified. The rather high proportions of late diagnoses are a clear cause for concern, especially if we bear in mind that squamous cell carcinomas form at the epithelial surface, giving rise to clearly visible early changes. Delays in diagnosis may be the “fault” of the patient (for not seeking medical attention in response to unusual oral symptoms) or of healthcare professionals (for not performing sufficiently detailed analyses of early lesions).
-Exfoliative oral cytology
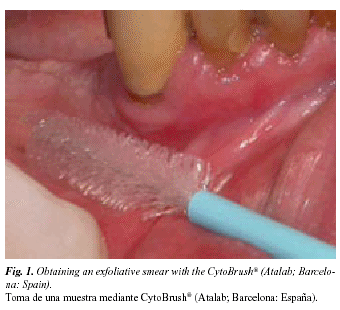
Exfoliative oral cytology can be defined as the obtention and characterization of cells from the surface of the oral mucosa. The cells may be detached naturally (as in mouthwash sampling) or artificially (as in cytobrush sampling). Cytobrush sampling (Fig. 1) is more frequently used, since it maximizes the number of cells obtained, and facilitates their uniform distribution onto the microscope slide, thus probably improving sensitivity (9). Whether based on mouthwash or cytobrush sampling, exfoliative oral cytology is a simple, non-aggressive, and relatively painless technique that is readily accepted by patients.
Characterization is traditionally based on microscopic examination of fixed and stained cell smears (6). Unfortunately, such methods show low sensitivity (i.e. a high proportion of false negatives) in the diagnosis of oral cancers (7). This low sensitivity is attributable to various factors, including inadequate sampling, a high risk of procedural errors, and the need for subjective interpretation of the findings (8). More recently, however, the continuing development of automated cytomorphometric methods, DNA content determination methods, immunocytochemical methods for detection of tumour markers, and diverse molecular-level analyses has contributed to renewed interest in exfoliative cytology procedures for the diagnosis of oral cancer. In what follows, developments in these areas are briefly reviewed.
-Cytomorphometry
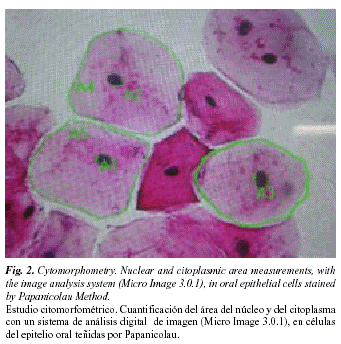
Ogden et al. (10) suggest that quantitative techniques, based on the evaluation of parameters such as nucleus area (NA), cytoplasm area (CA), and nucleus-to-cytoplasm area ratio (NA/CA), may increase the sensitivity of exfoliative cytology for early diagnosis of oral cancers, since these techniques are precise, objective and reproducible (Fig. 2).
Cowpe et al. (11) demonstrated that exfoliative cytology is capable of detecting malignant changes, through estimation of NA/CA using the planimeter method in Papanicolau-stained smears. This study, published in 1985, concluded that 50 cells were sufficient to provide indication of malignant changes. Since then, a number of studies have been carried out using the technique described by these authors to evaluate the influence of diverse systemic and external factors on NA, CA and NA/CA. In these studies planimeters have been replaced by semiautomatic image analysis techniques, which are faster, more accurate and more reproducible (12,13).
Cowpe et al. (14) found that tissues undergoing malignant transformation typically show a reduction in CA before the reduction in NA. They also suggested that samples of healthy mucosa from the same patient provide the best controls. Ramaesh et al. (15) used cytomorphometric techniques to assess nucleus diameter (ND) and cytoplasm diameter (CD) in normal oral mucosa, in dysplastic lesions and in squamous cell carcinomas. They found that CD was highest in normal mucosa, lower in dysplastic lesions, and lowest in SCCs. By contrast, ND was lowest in normal mucosa, higher in dysplastic lesions, and highest in SCCs. These studies suggest that reduced nucleus size and increased cytoplasm size are useful early indicators of malignant transformation, and thus suggest that exfoliative cytology is of value for monitoring clinically suspect lesions and for early detection of malignancy.
-Nuclear DNA content
Static cytometry permits the quantification of DNA content in cells obtained by exfoliative cytology. However, routine haematoxylin-eosin staining is inadequate for this purpose, and special techniques are required to ensure that staining intensity is in proportion to DNA content. The Feulgen reaction meets this criterion, since it is a stoichiometric procedure: in other words, each fixed molecule of Schiff’s reagent corresponds to a constant and equivalent portion of the DNA molecule. The great advantage of this procedure is that staining intensities (and thus DNA contents) can be determined automatically by spectrophotometry or densitometry and digital image analysis (16).
Cowpe et al. (11) have demonstrated that clinically normal mucosa in healthy patients shows a diploid DNA profile, while malignant lesions typically show an abnormal profile (17). However, and although the majority of oral malignant lesions show abnormal profiles, some may be diploid (18,19). Quantification of DNA content in conjunction with cell and nuclear morphometry appears to be a more discriminatory approach for distinguishing premalignant and malignant from non-malignant lesions. Remmerbach et al. (20), in a prospective study of the value of exfoliative cytology for the early diagnosis of oral cancer, analysed DNA content and cytomorphometric data for suspect lesions. They found that joint use of these approaches gave 98% sensitivity and 100% specificity for detection of malignancy, and concluded that DNA cytometry in conjunction with cytomorphometry is a sensitive, specific and objective procedure for the early identification of neoplastic cells in oral smears. Ogden et al. (21) also demonstrated the utility of DNA cytometry for monitoring malignant lesions after definitive treatment, with the aim of detecting recurrence. Recently, Sudbo et al. (22) showed that DNA content in tissue sections is of value for diagnosis of leucoplakias of the oral cavity. Malignant transformation rates for diploids, tetraploids and aneuploids were 3%, 60% and 84% respectively. In smears from leucoplakias and lichens that subsequently became malignant, Doseva et al. (23) found hypodiploid or hyperdiploid rather than diploid DNA profiles. Schimming et al. (24) evaluated the correlation between DNA distribution and the clinical-pathological characteristics of squamous cell carcinomas of the oral cavity. They found that non-diploid tumours had significantly more advanced stage, higher metastasis rate, and lower patient survival rate. The quantification of DNA content thus seems to be useful for predicting the behaviour of potentially malignant lesions, for establishing prognosis of malignant lesions, and for detecting post-treatment recurrences.
-Immunohistochemical identification of tumour markers
The identification of tumoral markers, notably cytokeratins, in smears from the oral cavity has attracted considerable interest. Cytokeratin expression profile provides useful information on cell differentiation status (25), but its potential for early diagnosis of oral cancer is limited, since there is no cytokeratin marker present in all malignant lesions but not in normal mucosa (26). However, certain cytokeratins, such as K8 and K19 (27), are useful if not definitive indicators of malignancy, particularly if their presence is interpreted in conjunction with other information, such as DNA profile (28).
Mutation of the tumour suppressor gene p53 is another frequent genomic change in human cancers (29). According to most studies, p53 is not present in normal oral mucosa (30-33), but is detectable by immunohistochemical methods in SCCs and potentially malignant lesions of the oral mucosa (34). Likewise, p53 has been detected in cells of malignant tumours obtained by exfoliative cytology, but not in normal mucosa (35).
However, immunohistochemical detection of p53 cannot be taken as definitive indication of a p53 mutation, since immunohistochemistry may likewise detect wild-type p53 (36). In addition, p53 mutations are present in only about 50% of squamous cell carcinomas of the oral cavity; this, together with the fact that p53 expression is only seen at advanced stages of carcinogenesis (37), means that early diagnosis of oral cancers on the basis of p53 detection in oral mucosa smears is not a useful option, since exfoliative cytology does not obtain basal epithelial cells (38).
In conclusion, then, we still lack specific tumoral markers present in all malignant lesions of the oral cavity but absent from benign lesions and normal mucosa (10).
-Molecular analyses
In recent decades we have seen a dramatic switch from histopathological to molecular methods of disease diagnosis (39), and exfoliative cytology has gained importance as a rapid and simple method for obtaining DNA samples. Loss of heterogeneity (LOH) and other molecular changes indicative of oral carcinogenesis can be readily identified in exfoliated cells (40-42). Huang et al. (43) used PCR (polymerase chain reaction) techniques to amplify DNA from exfoliation samples from oral carcinomas, for analysis of restriction-fragment length polymorphisms (RFLPs). They found that 66% of the tumours studied showed LOH at one position in the p53 sequence, while 55% showed LOH at some other location (43).
PCR and RFLP analysis have also been used for the detection of microsatellite markers, i.e. short repetitive DNA sequences. Microsatellite mutations, LOH or instability (MI) are all characteristic of the squamous cell carcinomas of head and neck, and can thus be used as molecular markers of malignancy. Nunes et al. (44) performed a microsatellite analysis of cells sampled from the oral cavity of oral and oropharyngeal cancer patients by exfoliative cytology and by mouthwash, finding LOH in 84% of samples, though with differences depending on tumour stage. These authors suggested that techniques of this type may be useful for early diagnosis and for patient monitoring.
In a similar study, Spafford et al. (45) identified genetic alterations (LOH or MI) in all of the malignant lesions of the oral cavity included in their sample. Conversely, none of their healthy patients showed such alterations, indicating the very high specificity of these methods.
Molecular analysis is set to become an essential technique in the diagnosis and management of oral cancer (46). Foreseeably, the use of molecular markers will permit detection of malignant changes before these become detectable clinically, and will facilitate monitoring of tumour progression and response to treatment. Techniques of this type may also be useful in population preventive programmes.
CONCLUSIONS
Exfoliative cytology is becoming increasingly important in the early diagnosis of oral cancers, as a procedure for obtaining cell samples that can then be analysed by sophisticated diagnostic techniques such as cytomorphometry, DNA cytometry, and molecular analyses. Exfoliative cytology is a simple and rapid, non-aggressive and relatively painless: it is thus well accepted by patients and suitable for routine application in population screening programmes, for early analysis of suspect lesions, and for pre-and? post-treatment monitoring of confirmed malignant lesions.
REFERENCES
1. Wood NK, Sawyer DR. Cáncer oral. En Wood NK, Goaz PW, eds. Diagnóstico diferencial de las lesiones orales y maxilofaciales. Harcourt Brace: Barcelona 1998. p. 587-95. [ Links ]
2. Serra Majen L, Ramón Torrel JM. Cáncer oral: Epidemiología y prevención. En: Cuenca Sala E, Manau Navarro C, Serra Majen L, eds. Odontología preventiva y comunitaria. Barcelona: Masson; 2003. p. 173-93. [ Links ]
3. Beeken SW, Krontiras H, Maddox WA, Peters GE, Soong S, Urist MM. T1 and T2 squamous cell carcinoma of the oral tongue: prognostic factors and the role of elective lymph node dissection. Head and neck 1999;21:124-30. [ Links ]
4. Sciuba JJ. Oral cancer and its detection. History-taking and the diagnostic phase management. JADA 2001;32:12s- 8s. [ Links ]
5. Silverman S Jr. Oral cancer. 4ª ed. Hamilton: American Cancer Society, BC Decker; 1998. p. 25-33. [ Links ]
6. Langlois CC, Devildos LR, Oliveira GL, Aver-Araújo LM, eds. Diagnóstico histopatológico. Manual de aulas práticas. 3 ed. Pelotas: Faculdade de Odontología – UFPel; 1993. p. 25-31. [ Links ]
7. Dabelsteen E, Roed-Petersen B, Smith CJ, Pindborg JJ. The limitations of exfoliative cytology for the detection of epithelial atypia in oral leukoplakias. Br J Cancer 1971;25:21- 4. [ Links ]
8. Sugerman PB, Savage NW. Exfoliative cytology in clinical oral pathology. Aust Dent J 1996;41:71-4. [ Links ]
9. Jones CJ, Pink FE, Sandow PL, Stewart CM, Migliorati CA, Baughman RA. The citobrush cell colector in oral cytology. Oral Surg Oral Med Oral Pathol 1994;77:101-7. [ Links ]
10. Ogden GR, Cowpe JG, Wight AJ. Oral exfoliative cytology: review of methods of assessment. J Oral Pathol Med 1997;26:201- 5. [ Links ]
11. Cowpe JG, Longmore RB, Green MW. Quantitative exfoliative cytology of normal oral squames: An age, site and sex related survey. J R Soc Med 1985; 78:995-1004. [ Links ]
12. Cowpe JG, Green MW, Ogden GR. Quantitative cytology of oral smears- a comparison of two methods of measurement. Analyt Quant Cytol Histol 1991; 13:11-5. [ Links ]
13. Cowpe JG, Ogden GR, Green MW. Comparison of planimetry and image analysis for the discrimination between normal and abnormal cells in cytological smears of suspicious lesions of the oral cavity. Cytopathol 1993;4:27-36. [ Links ]
14. Cowpe JG, Longmore RB, Green MW. Quantitative exfoliative cytology of abnormal oral mucosal smears. J R Soc Med 1988;81:509-13. [ Links ]
15. Ramaesh T, Mendis BRRN, Ratnatunga N, Thattil RO. Cytomorphotometric analysis of squames obtained from normal oral mucosa and lesions of oral leukoplakia and squamous cell carcinoma. J Oral Patol Med 1998;27:83- 6. [ Links ]
16. García del Moral R, Quesada MJ, Ruiz Avila I. Histoquímica de proteínas, aminas biógenas y ácidos nucleicos. En. García del Moral R, eds. Laboratorio de anatomía patológica. 1ª ed. Madrid: McGraw-Hill – Interamericana de España; 1993. p. 245- 63. [ Links ]
17. Tucker JH, Cowpe JG, Ogden GR. Nuclear content and morphometric characteristics of normal, premalignant, and malignant oral smears. Anal Cell Pathol 1994, 6: 117-28. [ Links ]
18. Franzen G, Klintenberg C, Olofsson J, Risberg B. DNA measurement: An objective predictor of responsee to irradiation? A review of 24 squamous cell carcinomas of the oral cavity. Br J Cancer 1986;53:643-51. [ Links ]
19. Tytor M, Franzen G, Olofsson J, Brunk U, Nordenskjold B. DNA content, malignancy grading and prognosis in T1 and T2 oral cavity carcinomas. Br J Cancer 1987;56:643-51. [ Links ]
20. Remmerbach TW, Weidenbach H, Pomjanski N, Knops K, Mathes S, Hemprich A, et al. Cytologic and DNA-cytometric early diagnosis of oral cancer. Anal Cell Pathol 2001;22:211-21. [ Links ]
21. Ogden GR, Cowpe JG. Quantitative cytomorphometric analysis as an aid to detection of recurrent oral cancer. Br J Oral Maxillofac Surg 1989;27:224-8. [ Links ]
22. Sudbo J, Kildal J, Risberg B, Koppang HS, Danielsen HE, Reith A. DNA content as a prognostic marker in patients with oral leukoplakia. N Engl J Med 2001;344:1270-8. [ Links ]
23. Doseva D, Cristov K, Kristeva K. DNA content in reactive hiperplasia, precancerosis, and carcinomas of the oral cavity. A cytophotometric study. Acta Histochem 1984;75:113-9. [ Links ]
24. Schimming R, Hlawitschka M, Haroske G, Eckelt U. Prognostic relevance of DNA image cytometry in oral cavity carcinomas. Anal Quant Cytol 1998; 20:43-51. [ Links ]
25. Lane EB, Alexander CM. Use of keratin antibodies in tumour diagnosis. Seminar of Cancer Biology 1990;1:165-79. [ Links ]
26. Ogden GR, Chisholm DM, Adi M, Lane EB. Cytokeratin expresion in oral cancer and its relationship to tumour differentation. J Oral Pathol Med 1993; 22:82-6. [ Links ]
27. Ogden GR, McQueen S, Chisholm DM, Lane EB. Keratin profiles of normal and malignant oral mucosa using exfoliative cytology. J Clin Pathol 1993; 46:352- 6. [ Links ]
28. Ogden GR, Cowpe JG, Chisholm DM, Lane EB. DNA and keratin analysis of oral exfoliative cytology in the detection of oral cancer. Oral Oncol, Eur J Cancer 1994;30B,405-8. [ Links ]
29. Rosel R. Molecular origens of human cancer. En: Rosel R, Abad A, Monzó M, Barnadas A, eds. Manual de oncología clínica y molecular. Badalona: Arán Ediciones; 2000. p. 1-15. [ Links ]
30. Langdon JD, Partidge M. Expresión of the tumour supresor gene p53 in oral cancer. Br J Oral Maxillofac Surg 1992;30:214- 20. [ Links ]
31. Ogden GR, Kiddie RA, Lunny DP, Lane DP. Assessment of p53 protein expresión in normal, benign and malignant oral lesions. J Pathol 1992;166: 389- 94. [ Links ]
32. Warnakulasuriya KA, Johnson NW. Expresión of p53 mutant nuclear phosphoprotein in oral carcinoma and potentially malignant oral llesions. J Oral Pathol Med 1992;21:404-8. [ Links ]
33. Kaur J, Srivastava A, Ralhan R. Overexpresión of p53 protein in betel- and tobacco related human oral dysplasia anss squamous cell carcinoma in India. Int J Cancer 1994;58:340-5. [ Links ]
34. Raybaud-Diogene H, Tetu B, Morency R, Fortin A, Monteil RA. p53 over-expressión in head and neck squamous cell carcinoma: a review of the literature. Eur J Cancer B Oral Oncol 1996;32B:143-9. [ Links ]
35. Ogden GR, Cowpe JG, Chisholm DM, Lane DP. P53 immunostainning as a marker for oral cancer in diagnostic cytopathology – preliminary report. Cytopathol 1994;6:117-28. [ Links ]
36. López-Martínez M, Anzola M, Cuevas N, Aguirre JM, Martínez de Pancorbo M. Aplicaciones clínicas del diagnóstico de las alteraciones de p53 en el carcinoma escamoso de cabeza y cuello. Med Oral 2002;7:108-20. [ Links ]
37. Ogden GR, Kiddie RA, Lunny DP, Lane DP. Assessment of p53 protein expresion in normal, benign and malignant oral mucosa. J Pathol 1992;166: 389-94. [ Links ]
38. Ogden GR, Leigh I, Chisholm DM, Cowpe JG, Lane EB. Exfoliative cytology of normal oral mucosa- assessing basal cel keratin phenotype. Acta Cytol 1996;40:933-6. [ Links ]
39. Ogden GR. The future role for oral exfoliative cytology- bleak or bright? Oral Oncol 1997;33:2-4. [ Links ]
40. Rossin Mp, Cheng X, Poh C, Lam WL, Huang Y, Lovas J et al. Use o allelic loss to predict malignant risk for low-grade oral epithelial dysplasia. Clin Cancer Res 2000;6:357-62 [ Links ]
41. Mao L, Lee JS, Fan YH, Ro JY, Batsakis JG, Lippman S, et al. Frequent microsatellite alterations at chromossome 9p21 and 3p14 in oral premalignant lesions and their value in cancer risk assessment. Nat Med 1996;2:682-5. [ Links ]
42. Partridge M, Pateromchelakis S, Phillips E, Emilion GG, A´Hern RP, Langdon JD. A case control-study confirms that microsatellite assay can identify patients at risk of developin squamous cell carcinooma within field cancerization. Cancer Res 2000;60:3893-8. [ Links ]
43. Huang MF, Chang YC, Liao PS, Huang TH, Tsay CH, Chou MY. Loss of heterozygosity of p53 gene of oral cancer detected by exfoliative cytology. Oral Oncol 1999;35:296-301. [ Links ]
44. Nunes DN, Kowalski LP, Simpson AJ. Detection of oral and oropharyngeal cancer by microsatelite analysys in mouth washes and lesions brushings. Oral Oncol 2000;36:525-8. [ Links ]
45. Spafford MF, Koch WM, Reed AL, Califano JA, Xu LH, Eisenberger CF, et al. Detection of head and neck squamous cell carcinoma among exfoliated oral mucosa cells by microsatelliite análisis. Clin Cancer Res 2001;7:607-12. [ Links ]
46. Epstein JB, Zhang L, Rosin M. Advances in the diagnosis of oral prema-lignant and malignant lesions. J Can Dent Assoc 2002;68:617-21. [ Links ]











 text in
text in