My SciELO
Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Medicina Oral, Patología Oral y Cirugía Bucal (Internet)
On-line version ISSN 1698-6946
Med. oral patol. oral cir.bucal (Internet) vol.11 n.1 Jan./Feb. 2006
ORAL SURGERY
Physiological bases of bone regeneration I. Histology and physiology of bone tissue
Isabel Fernández-Tresguerres Hernández-Gil 1, Miguel Angel Alobera Gracia 1,
Mariano del Canto Pingarrón 1, Luis Blanco Jerez 2
(1) Profesor Titular Interino, MD. PhD. DDS, Departamento de Ciencias de la Salud III,
Facultad de Ciencias de la Salud, Universidad Rey Juan Carlos, Alcorcón
(2) Profesor Titular, MD. PhD. DDS. Departmento de Medicina y Cirugía Bucofacial,
Facultad de Odontología, Universidad Complutense. Madrid
ABSTRACT
Bone is the only body tissue capable of
regeneration, allowing the restitutio ad integrum following trauma. In the event
of a fracture or bone graft, new bone is formed, which following the remodeling
process is identical to the pre-existing.
Bone is a dynamic tissue in constant formation and resorption. This balanced
phenomena, known as the remodeling process, allows the renovation of 5-15% of
the total bone mass per year under normal conditions (1). Bone remodeling
consists of the resorption of a certain amount of bone by osteoclasts, likewise
the formation of osteoid matrix by osteoblasts, and its subsequent
mineralization. This phenomenon occurs in small areas of the cortical bone or
the trabecular surface, called "Basic Multicellular Units" (BMU).
Treatment in Traumatology, Orthopedics, Implantology, and Maxillofacial and Oral
Surgery, is based on the biologic principals of bone regeneration, in which
cells, extracellular matrix, and osteoinductive signals are involved.
The aim of this paper is to provide an up date on current knowledge on the
biochemical and physiological mechanisms of bone regeneration, paying particular
attention to the role played by the cells and proteins of the bone matrix.
Key words: Bone, regeneration, resorption, osteogenesis.
RESUMEN
El hueso es el único tejido del organismo capaz de
regenerarse, permitiendo la restitutio ad integrum tras el trauma. Cuando se
produce una fractura, se coloca un implante osteointegrado o se realiza un
injerto para aumentar el sustrato óseo antes de la inserción de implantes, lo
que se pretende es la regeneración ósea, es decir, la formación de hueso nuevo
que, tras un proceso de remodelado, sea idéntico al preexistente.
El hueso es un tejido dinámico en constante formación y
reabsorción. Este fenómeno equilibrado, denominado proceso de remodelado,
permite la renovación de un 5-15 % del hueso total al año en condiciones
normales (1). El remodelado óseo consiste en la reabsorción de una cantidad
determinada de hueso llevada a cabo por los osteoclastos, así como la formación
de la matriz osteoide por los osteoblastos y su posterior mineralización. Este
fenómeno tiene lugar en pequeñas áreas de la cortical o de la superficie
trabecular, llamadas "unidades básicas de remodelado óseo".
La actuación terapéutica en los campos de la Traumatología y
Ortopedia, Cirugía Oral y Maxilofacial e Implantología, se asienta sobre los
principios biológicos de la regeneración ósea, en los que están implicados
células, matriz extracelular y señales osteoinductivas.
El objetivo de este trabajo es realizar una puesta al día de
los conocimientos actuales sobre los mecanismos bioquímicos y fisiológicos de la
regeneración ósea, resaltando de manera especial el papel que en ella juegan las
células y las proteínas de la matriz ósea.
Palabras clave: Hueso, regeneración, reabsorción, osteogénesis.
Introduction
From the histological point of view, bone is a highly vascularized and innervated, mineralized conjunctive tissue, which is structured in lamellae of calcified osteoid matrix. The arrangement of these lamellae determines whether the bone is cortical or cancellous. Both are composed of osteons. Cortical or compact bone, containing osteocytes, is arranged concentrically around Haversian Canals. Cancellous or trabecular bone is formed by a network of bone lamellae, delimiting areolar cavities inside which the bone marrow is found (2). Both cortical and trabecular bone contain specialized cells, organic matrix and mineral phase.
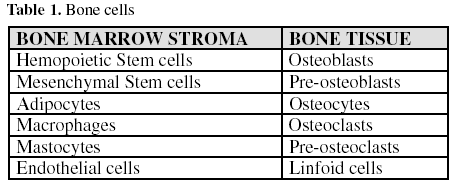
1. Bone Cells
Various cell types co-exist in bone (table 1). Bone cells are found within the bone tissue itself or in the conjunctive stroma of the bone marrow, which is rich in mesenchymal stem cells. From the studies by Friedenstein in 1976 it is known that five distinct cell types can originate from these stem cells: fibroblasts, osteoblasts, chondroblasts, adipocytes and myoblasts (3), in response to different molecular signals which initiate the activation cascade of different genes.
1.1 Osteoblastic differentiation
A/- Growth factors and genetics: It is currently known that the differentiation towards the osteoblastic line is controlled by genes related to the Hedgehog family, of which the most well known are: Ihh (Indian hedgehog) and Shh (Sonic hedgehog) (4,5). Also essential are the transcription factor Cbfa1 (core-binding factor a-1, also known as Runx2) (6,7,8,9) and the bone morphogenetic proteins (BMPs), which constitute the most powerful regulators of osteoblastic differentiation from the mesenchymal stem cells (4).
B/- Differentiation markers: As the precursor cells differentiate they express proteins specific to their function, or markers, in the cellular membrane. The expression of Cbfa1 is the first indication of osteogenic differentiation (4), the maximum level of which is reached in the pre-osteoblasts. Collagen I and osteopontin (OPN) are expressed early on in osteoprogenitor cells. In the same way alkaline phosphatase (ALP) is a surface protein that could participate in the regulation of proliferation, migration and differentiation of the osteoblastic cells. Bone sialoprotein (BSP) and osteocalcin (OCN), are pre-osteoblast to osteoblast differentiation markers and appear when mineralization begins. The expression of these proteins is especially useful as osteogenic markers in the final stages of osteoblastic differentiation.
1.2 The osteoblast
Osteoblasts are large cells (20-30 µm), in the form of a polyhedron, with a basophilic cytoplasm, and with a substantial rough endoplasmic reticulum and Golgi apparatus. They originate from the mesenchymal stem cells of the bone marrow, endosteum, periosteum, and perivascular pericytes (10). They emit cytoplasmic processes towards the matrix, which communicate with the osteocyte network and neighbouring osteoblasts. The osteoblasts and the osteocytes communicate with each other by transmembrane proteins or integrins, which act as a link between cells or between a cell and the extracellular matrix, allowing the passage of messengers such as calcium, cytokines and prostaglandins. In these cells the intercellular connection is Connexin 43 (11).
The osteoblasts synthesize the organic matrix or osteoid material at a rate of 2 to 3 µm per day, and express a characteristic enzyme, alkaline phosphatase (ALP), which permits mineralization at a rate of 1-2 µm per day. It is now known that they: 1 - synthesize the collagen and non-collagen proteins of the organic bone matrix, 2 - direct the arrangement of the extracellular matrix fibrils, 3 - contribute to the mineralization of the osteoid material, due to the alkaline phosphatase, 4 - mediate in the resorption carried out by the osteoclasts, through the synthesis of specific cytokines (12) and 5 - synthesize growth factors.
The average lifespan of human osteoblasts is from 1 to 10 weeks, at the end of which they can disappear through apoptosis, become transformed into bone lining cells or into osteocytes (15%) (13). Both types of cells represent more advanced stages of maturation. The bone lining cells are elongated and flat, with spindle shaped nuclei, and scarce organelles. They can express the previously mentioned osteoblastic markers such as bone sialoprotein, osteopontin, osteonectin, and alkaline phosphatase as well as parathyroid hormone receptor (PTH). They remain along the endosteal surface, forming with the endosteum a protective layer to the bone surface, which plays an important role in the activation of bone remodeling.
1.3 The osteocyte
Once the matrix is mineralized, some osteoblasts remain trapped within, becoming transformed into osteocytes. The osteoblasts, osteoclasts and bone lining cells are found on the bone surface, while the osteocytes are on the interior. The osteocytes are the most abundant cells in bone (10 times the osteoblasts). They are stellate and are found in the interior of lacunae, the cytoplasmic processes communicate with each other through bone canaliculi filled with extracellular bone fluid. In this way, the osteocytes organize themselves into a syncytium of interconnected cells which forms a single structure, with the advantage that there is a large contact surface both on the inside and towards the bone surface, assuring the supply of oxygen and nutrients. When trauma occurs in the bone, the cessation in the blood supply causes hypoxia and necrosis of the osteocytes situated further than 0.1 mm from an intact capillary vessel (14).
The osteocytes also participate in the synthesis and mineralization of the osteoid matrix, but it is believed that their principal function is to control bone remodeling, detecting the mechanical variations of the loads, a phenomena known as mechanotransduction (15).
Osteocytes constitute the final stage of the osteoblastic line and are incapable of self-renewal. They have the same markers as the osteoblasts, but also have a specific marker, CD44, a membrane receptor, strongly expressed in osteocytes and negative in osteoblasts and bone lining cells.
1.4 The osteoclast
Osteoclasts are the cells responsible for resorption. These are large cells (100 µm), multinucleate, rich in mitochondria and vacuoles. The osteoclasts contain tartrate-resistant acid phosphatase (TRAP), which permits the dephosphorylation of the proteins, the activity of which is used for its identification both in vivo and in vitro. In addition they possess receptors for calcitonin.
The osteoclasts originate from the bone marrow hematopoietic stem cells known as Granulocyte-Macrophage Colony-Forming Units (GM-CFU), precursors of macrophages and monocytes (16).
Osteoclasts have two special features in the membrane: a ruffled border, where resorption takes place, and a clear area rich in microfilaments, with integrins that serve as an anchor to the matrix. To this end, the osteoclasts move towards the area to be resorbed and then immediately adhere to the mineralized bone surface with the ruffled border and sealing the edges of the area with the integrins. The osteoclast integrin, particularly avβ3, recognizes the Arg-Gly-Asp (RGD) sequence in the collagen and other proteins of the osteoid matrix. At this level the pH is acid since they secrete acids (H+) generated by carbonic anhydrase II and proteolytic enzymes such as collagenases, metalloproteases, cathepsin K, glucuronidase, etc. (16), which initiate bone resorption by the solubilization of first the organic and then the mineral matrix.
With respect to osteoclastogenesis, it is currently known that the osteoblasts are fundamental to the formation of osteoclasts. Thus, the Macrophage Colony-Stimulating Factor (M-CSF) produced by the osteoblasts is required in the early phases of osteoclastogenesis for the formation of giant multinucleate cells. Present knowledge on osteoclastogenesis regulation is based on the existence of 3 key molecules: OPG (osteoprotegerin, a soluble protein synthesized by osteoblasts and pre-osteoblasts), RANKL (a ligand situated on the surface of the osteoblasts and pre-osteoblasts), and RANK (a receptor of the above, situated on the osteoclast and pre-osteoclast membranes). The RANKL (receptor activator of NFkB ligand) previously called ODF (osteoclast differentiation factor) (12, 17) is a transmembrane cytokine belonging to the tumor necrosis factor family (TNF) (18). The interaction between RANKL and its receptor RANK initiates osteoclastic activity and differentiation, increasing resorption. Likewise, the effects of RANKL both in vivo and in vitro are inhibited by osteoprotegerin (OPG) a circulating protein belonging to the superfamily of TNF receptors (12).
When OPG and RANKL bind together, the union between RANK and RANKL is inhibited, and thus the osteoclastic differentiation is also inhibited. For this reason OPG, RANK and RANKL are important regulators of osteoclastogenesis.
2. Organic matrix
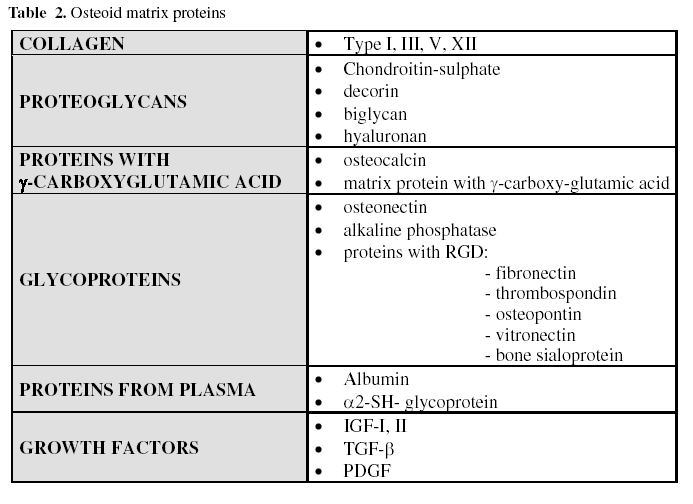
The organic matrix or osteoid material makes up a third of the bone mass. It is formed fundamentally by proteins, especially collagen (90%) (Table 2). The matrix plays an important role within the entire bone system, a fact which becomes evident when collagen disease, such as imperfect osteogenesis, appears. However, the extracellular mineralized matrix should now be considered as something more than simply a reservoir of calcium and phosphorous, since it constitutes a reserve of proteins that participate in the regulation of cellular differentiation and in the integrity and function of bone tissue (19).
A - Collagen
Ninety percent of the extracellular matrix (ECM) is made up of collagen, above all type I (>95%) and type V (<5%). The presence of small amounts of collagen type III has also been found, related to Sharpey fibers, and type XII, formed under mechanical stress. In the collagen molecule the Arg-Gly-Asp (RGD) sequence is found, which is recognised by the surface integrins of the bone cells (20). Characteristically they contain the hydroxylysine and hydroxyproline amino acids, the latter being a specific marker of all the collagen phenotypes and its urinary secretion values being directly related to the bone resorption rate (21). The collagen fibers are established by means of hydrogen bridges between amino acids and through the formation of pyridinoline bridges between lysines and hydroxylysines. However, collagen has no great affinity for calcium, for this reason other proteins are involved in mineral deposition.
B - Non-collagen proteins: Among which are highlighted:
B.1 Proteoglycans: are large molecules and make up 10% of the non-collagen proteins. In the osteoid matrix there are four types of proteoglycans: Hyaluronan and chondroitin-sulphate: are large molecules, and take part in the initial stages of bone morphogenesis. Biglycan and decorin: are smaller molecules, and appear in the next phases of bone formation.
B.2 Proteins with γ- carboxyglutamic acid: These are osteocalcin (OCN) and the matrix protein with γ- carboxyglutamic acid. This is an amino acid which binds calcium and requires vitamin K for its synthesis.
Osteocalcin is a small matrix protein synthesized by osteoblasts and platelets. Dependent on vitamins D and K, it represents 15% of the non-collagen matrix proteins and contains 3 molecules of γ-carboxyglutamic acid. Its plasmatic levels have been considered as one of the biochemical markers of osteogenesis, being related with the number and activity of the osteoblasts.
B.3 Glycoproteins: These are osteonectin, alkaline phosphatase, and proteins with the RGD tripeptide (Arg-Gly-Asp).
Osteonectin is a glycoprotein with a strong affinity for collagen type I, calcium and hydroxyapatite. It represents 25% of the non-collagen proteins. It is thought to play a role in the regulation of cellular adhesion between the matrix and the cells. In bone it is necessary for normal mineralization.
Alkaline phosphatase is an enzyme that liberates inorganic phosphate from phosphoric esters, and is necessary for mineralization. Various isoenzymes exist; the bone one is considered a good marker of osteoblastic activity.
There are fundamentally five proteins with the RGD tripeptide, also called SIBLINGS (Small Integrin-Binding Ligand, N-linked Glycoprotein): osteopontin, bone sialoprotein, fibronectin, thrombospondin and vitronectin. These glycoproteins are fundamental to bone regeneration and remodeling processes, with an Arg-Gly-Asp (RGD) sequence which is recognized by the osteoblast and osteoclast integrins (avβ3, among others). They also act as bone cell surface receptors, allowing the adhesion of the cells to the extracellular matrix, and activating signals.
B.4 Proteins originating from plasma: a greater proportion of these are found in the organic bone matrix than in plasma. They are albumin and a2-SH-glycoprotein, probably related with the deposition of calcium in the osteoid matrix.
B.5 Growth factors: these are polypeptides, synthesized within the bone itself or derived from other locations (liver, platelets, etc.), and which take part in the autocrine or paracrine differentiation, growth and proliferation of the cells (table 2) (22).
3. Mineral phase
Finally, the mineral component of bone represents 65% of the bone mass. It is formed by calcium, phosphate and carbonate (in proportions of 10:6:1) in the form of small hydroxyapatite crystals Ca10 (PO4)6(OH)2 and, in smaller quantities, magnesium, sodium, potassium, manganese and fluoride. The plasma is supersaturated with calcium and phosphorous with respect to hydroxyapatite, so there must be substances which inhibit mineralization. The proteins with adhesive capacity favor mineralization, while the proteoglycans, magnesium, ATP and pyrophosphates act as inhibitors.
4. Bone regeneration
Tissue regeneration is the solution achieved by the restitutio ad integrum of tissue following trauma, in contrast to repair, where scar tissue is formed having different characteristics from the original. In this regard, bone is the only tissue in the body, with the exception of embryonic, which is fully restored following an injury (1). Bone regeneration creates a response in which blood vessels, cells and extracellular matrix are involved. The importance of blood vessels in osteogenesis is known from studies by Trueta (23). Following trauma, an inflammatory response and an initial hematoma is produced, with red blood cells, platelets and fibrin. The cells release interleukins and growth factors, setting off the migration of lymphocytes, macrophages, osteoclast precursors and stem cells. These molecular signals promote the differentiation towards endothelial cells, fibroblasts, chondroblasts and osteoblasts, creating a new fibrovascular tissue which replaces the initial coagulate. This is all regulated by a series of complex interactions between growth factors, hormones and cytokines. The vascular supply, proteic synthesis and mineralization are all fundamental to this process.
![]() Correspondence
Correspondence
Dra. Isabel Fernández-Tresguerres Hernández-Gil
Facultad de Ciencias de la Salud, Avda de Atenas s/n,
Alcorcón, 28922 Madrid.
Teléfono: 91 4888941.
E-mail: isatresguerres@yahoo.es
Recibido: 2-08-2004
Aceptado: 14-08-2005
References
1. Davies JE, Hosseini MM. Histodinamics of endosseous would healing. En: Davies JE ed. Bone Engineering. Toronto: Davies JE ed.; 2000. p. 1-14. [ Links ]
2. Wheater PR, Burkitt HG, Daniels VG. Functional Histology. New York: Churchill Livingstone ed.; 1987. p. 142-60. [ Links ]
3. Friedenstein AJ. Precursor cells of mechanocytes. Int Rev Cytol 1976;47:327-55. [ Links ]
4. Yamaguchi A, Komori T, Suda T. Regulation of osteoblast differentiation mediated by Bone Morphogenetic Proteins, Hedgehogs, and Cbfa1. Endocr Rev 2000;21:393-411. [ Links ]
5. Aubin JE. Osteogenic cell differentiation. En: Davies JE ed. Bone Engineering. Toronto: Davies JE ed.; 2000. p. 19-30. [ Links ]
6. Heersche JNM. Mesenchymal stem cells and their involvement in bone remodeling, repair, and regeneration. En: Zarb G, Leckholm U, Albrektsson T, Tenenbaum H eds. Aging, Osteoporosis, and Dental Implants. Carol Stream: Quintessence Publishing Co.; 2002. p. 17-23. [ Links ]
7. Komori T, Yagi H, Nomura S, Yamaguchi A, Sasaki K, Deguchi K et al. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell 1997;89:755-64. [ Links ]
8. Ducy P, Zhang R, Geoffroy V, Ridall AL, Karsenty G. Osf2/Cbfa1: a transcriptional activator of osteoblasts differentiation. Cell 1997;89:747-54. [ Links ]
9. Hoshi K, Komori T, Ozawa H. Morphological characterization of skeletal cells in Cbfa1-deficient mice. Bone 1999;25:639-51. [ Links ]
10. Canfield AE, Doherty MJ, Ashton BA. Osteogenic potential of vascular pericytes. En: Davies JE ed. Bone Engineering. Toronto: Davies JE ed.; 2000. p. 143-51. [ Links ]
11. Civitelli R, Beyer EC, Warlow PM, Robertson AJ, Geist ST, Steinberg TH. Conexin 43 mediates direct intercellular communication in human osteoblastic cells networks. J Clin Invest 1993;91:1888-96. [ Links ]
12. Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang M-S, Luethy R et al. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell 1997;89:309-19. [ Links ]
13. Aubin JE, Liu F. The osteoblasts lineage. En: Bilezikian JP, Raisz LG, Rodan GA, eds. Principles of Bone Biology. San Diego, California: Academic Press;1996. p. 51-67. [ Links ]
14. Ham AW. Some histophysiological problems peculiar to calcified tissue. J Bone Joint Surg Am 1952;34:701. [ Links ]
15. Lanyon L. Osteocytes, strain detection, bone remodeling and remodeling. Calcified Tissue Int 1993;53:102-7. [ Links ]
16. Mundy GR. Cytokines and growth factors in the regulation of bone remodeling. J Bone Miner Res 1993;8:505-10. [ Links ]
17. Burgess TL, Quian Y, Kaufman S, Ring BD, Van G, Capparelli C et al. The ligand for osteoprotegerin (OPGL) directly activates mature osteoclasts. J Cell Biol 1999;145:527-38. [ Links ]
18. Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burguess TL et al. Osteoprotegerin ligand is a cytokine that regulates osteoclasts differentiation and activation. Cell 1998;93:165-76. [ Links ]
19. Young MF. Bone matrix proteins: more than markers. Calcif Tissue Int 2003;72:2-4. [ Links ]
20. Gehron Robey P, Fedarko NS, Hefferan TE, Bianco P, Vetter UK, Grzesik W et al. Structure and molecular regulation of bone matrix proteins. J Bone Miner Res 1993;8:483-7. [ Links ]
21. Schonau E, Rauch F. Markers of bone and collagen metabolism. Problems and perspectives in Pediatrics. Horm Res 1997;48:50-9. [ Links ]
22. Canalis E, Economides AN, Gazzerro E. Bone morphogenetic proteins, their antagonists, and the skeleton. Endocr Rev 2003;24:218-35. [ Links ]
23. Trueta J. The role of blood vessels in osteogenesis. J Bone Joint Surg Br 1963;45:402. [ Links ]











 text in
text in