Originals
Study of prescription-indication of methylphenidate in adults in a Healthcare Management Area
Estudio de prescripción-indicación de metilfenidato en adultos en un Área de Gestión Sanitaria
N Báez-Gutiérrez
1
, MC Saborido-Cansino
2
, A Sánchez-Pedrosa
2
1Servicio de Farmacia. Hospital Universitario Virgen del Rocío. Sevilla (España)
2Servicio de Farmacia. Área de Gestión Sanitaria Sur de Sevilla (España)
Summary
Introduction:
Even though in Spain methylphenidate is approved for attention deficit hyperactivity disorder (ADHD) in children older than 6 years, there are prescriptions in adult patients both for the treatment of ADHD and in other indications. The objective of this study is to analyse the adequacy of the prescription of methylphenidate in adult patients in the South of Seville Healthcare Management Area (SSHMA).
Methodology:
Retrospective observational prescription-indication study of the use of methylphenidate of all adult patients (>18 years) who had active methylphenidate prescriptions in September 2018.
Results:
We included 91 adult patients on treatment with methylphenidate with a mean age of 21 years (range 18-90 years). 67.03% were men. The diagnosis that most frequently motivated the prescription of methylphenidate was ADHD, which is the indication for which it is authorized. 36.26% of the patients presented indications not included in the technical data sheet. The mean daily dose prescribed was 36 mg (range 5-108 mg). 7 patients had prescribed doses higher than the maximum recommended.
Conclusions:
This study demonstrates different prescription patterns of methylphenidate in adults. Draws attention, the duration of treatment and non-suspension during adulthood. In addition, it is observed that the use of methylphenidate outside indications of technical data sheet is a common practice in adults.
Key Words: Methylphenidate; adults; prescription; indication
RESUMEN
Resumen
Introducción: A pesar de que en España el metilfenidato está aprobado para el trastorno por déficit de atención-hiperactividad (TDAH) en niños mayores de 6 años, existen prescripciones en pacientes adultos tanto para el tratamiento del TDAH como en otras indicaciones. El objetivo de este estudio es analizar la adecuación de la prescripción de metilfenidato en pacientes adultos en el Área de Gestión Sanitaria Sur de Sevilla.
Métodos:
Se realizó un estudio observacional retrospectivo y de corte transversal de utilización de medicamentos de tipo prescripción-indicación de todos los pacientes adultos (>18 años) que tenían activas prescripciones de metilfenidato en septiembre de 2018.
Resultados:
Se incluyeron 91 pacientes adultos en tratamiento con metilfenidato con una mediana de edad de 21 años (rango 18-90 años). Un 67,03% eran hombres. El diagnóstico que más frecuentemente motivó la prescripción del metilfenidato fue el TDAH, que es la indicación para la cual está autorizada. Un 36,26% de los pacientes presentaron indicaciones no incluidas en ficha técnica. La mediana de dosis diaria prescrita fue de 36 mg (rango 5-108 mg). 7 pacientes tenían prescritas dosis superiores a la máxima recomendada.
Conclusiones:
Este estudio demuestra diferentes patrones de prescripción de metilfenidato en adultos. Destacan la duración del tratamiento y la no suspensión durante la edad adulta. Además, se observa que el uso del metilfenidato fuera de indicaciones de ficha técnica es una práctica habitual en adultos.
Palabras clave: Metilfenidato; adultos; prescripción; indicación
INTRODUCTION
Methylphenidate is a psychostimulant that inhibits the reuptake of dopamine (DA) and norepinephrine (NE), increasing the amount of these monoamines in the synaptic cleft. Methylphenidate inhibits the reuptake of these monoamines by blocking their transporters: dopamine transporter (DAT) and noradrenaline transporter (NET), presenting greater affinity for DAT than for NET1.
Methylphenidate has structural and pharmacological similarity with drugs such as cocaine and D-amphetamine, so there is reason to suspect that it may have a potential for significant abuse2.
Currently in Spain, methylphenidate is only approved for attention deficit hyperactivity disorder (ADHD) in children from 6 years of age when other measures by themselves have proven to be insufficient3.
Treatment with methylphenidate is not indicated for all children with this syndrome and the decision to use the drug should be based on a very complete assessment of the severity and chronicity of the child's symptoms in relation to their age3.
There are different commercial presentations currently available on the market. The technical data sheet of some presentations (Equasym®, Medikinet® and Rubifen®) specifies that they are not indicated for adults. The rest of the presentations do not rule out this possibility for teenagers patients whose symptoms persist until adulthood and who have demonstrated a clear benefit. However, it is not appropriate to start treatment in adulthood3.
Regarding long-term use of methylphenidate (more than 12 months), the safety and efficacy of methylphenidate treatment have not been systematically evaluated in controlled studies. Treatment with methylphenidate should not be and is not required to be indefinite, it is usually suspended during or after puberty3.
In the last decades, the diagnosis of ADHD and the use of stimulants has increased significantly, reaching up to triple the prescription rate of methylphenidate4.
Other clinical uses, outside the technical specifications described in the bibliography, are:
Narcolepsy5 (indication approved by the FDA).
Depressive anxious disorder (obsessive compulsive disorder6, bipolar disorder6, major unipolar depressive disorder7, schizoid personality disorder8).
Disorder of the use of stimulants9 (detoxification).
Sleep disorders (obstructive sleep apnea10, idiopathic hypersomnia11).
Autism spectrum disorders12.
Cognitive enhancement13.
The use of medical stimulants such as methylphenidate to enhance intellectual capacity is increasing in some countries not only in patients with brain disorders but also in healthy individuals. Patients who consume cognition-enhancing drugs are at risk of developing dependence and suffer adverse effects due to the abuse of these substances13,14,15.
The aim of our study was to analyse the suitability of the prescription of methylphenidate in adult patients in the South of Seville Healthcare Management Area (SSHMA).
METHODS
Retrospective observational prescription-indication study of the use of methylphenidate in the South of Seville Healthcare Management Area. All adult patients (>18 years old) who had active methylphenidate prescriptions in September 2018 were included.
The following variables were collected: demographic (age and sex) and clinical (indication of treatment, total daily dose, posology, duration of treatment).
As outcome variables were collected: percentage of patients with off-label indications, percentage of patients with inadequate doses and percentage of patients with treatment duration of more than one year.
Patients with active prescription of methylphenidate from the SSHMA were obtained from the application LISTADOS®. The rest of the variables were collected from the digital clinical history (Diraya®) and from the prescription module (Prescripciones 5®).
RESULTS
Patients
91 adult patients on treatment with methylphenidate were included. The median age was 21 years old (range 18-90 years) 67.03% were men.
The greatest number of patients who consumed methylphenidate were between 18 and 25 years old (61.54%). A 12% of the cases were older than 60 years.
Indication study
The prescription indications for methylphenidate were collected in table 1. 36.26% of the patients presented indications not included in the technical data sheet.
Table 1. Indications of methylphenidate prescription
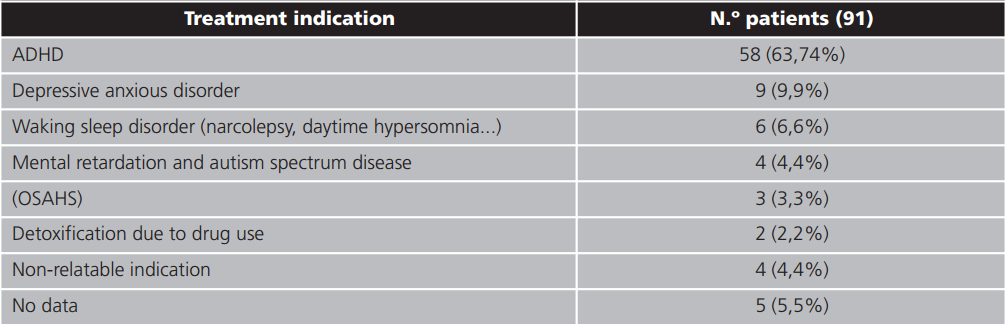
ADHD: attention deficit hyperactivity disorder; OSAHS: obstruvtive sleep apnea-hypopnea syndrome.
Dosage
The mean daily dose was 36 mg (range 5-108 mg) given in one or two daily doses. 7.7% of patients (7) had prescribed doses higher than the maximum recommended (60 mg day), 6 of them with a diagnosis of ADHD and one diagnosed with narcolepsy. Of these 7 patients, two had prescribed a total daily dose of 108 mg, another two 80 mg daily and the three remaining 72 mg daily.
Treatment duration
The duration of the treatment was measured approximately, since there are no electronic prescription records beyond the year 2003, the year in which the Recipe XXI was incorporated into the Andalusian Health System16.
The mean duration of treatment was 7.8 years (range 13.2-0.53 years). 96.7% of patients had been prescribed methylphenidate for more than a year.
DISCUSSION
The diagnosis that most frequently motivated the prescription of methylphenidate was ADHD, which is the indication for which it is intended. This states that CNS stimulants continue to be the first pharmacological line for this pathology. However, methylphenidate has not been approved in adults or the elderly, as there are no safety studies in these populations.
The increase in prescriptions and the prolongation of their duration is likely to cause an increase in adverse effects, despite the fact that most individuals with ADHD adequately use their medications17.
In the study population, the largest number of patients who consumed methylphenidate were included in the age group (18-25 years) who maintained the treatment initiated during childhood, which has not been suspended.
It was found that not all the prescribed doses were within the ranges recommended by the data sheet. Doses higher than these are associated with signs of generalized central CNS stimulation that can culminate in convulsions2. In our case, 7 patients had prescribed doses higher than those recommended.
CONCLUSIONS
This study demonstrates the different patterns in the prescription of methylphenidate in adults. Draws attention, the long duration of treatment with methylphenidate and the non-suspension of it during adulthood. In addition, it is observed that the use of methylphenidate outside indications of technical data sheet is a common practice in adults. These prescription patterns imply the need to incorporate strategies to minimize the risk of adverse reactions and the risk of misuse or abuse, particularly in the elderly.
Funding source: The present investigation has not received specific aid from agencies from the public sector, commercial sector or non-profit entities.
BIBLIOGRAPHY
1. Oakes HV, DeVee CE, Farmer B, Allen SA, Hall AN, Ensley T, et al. Neurogenesis within the hippocampus after chronic methylphenidate exposure. J Neural Transm (Vienna). 2019 Feb;126(2):201-209.
[ Links ]
2. Kollin SH, MacDonald EK, Rush CR. Assessing the abuse potential of methylphenidate in nonhuman and human subjects A review. Pharmacology, Biochemistry and Behavior. 2001 Mar;68(3):611-27.
[ Links ]
3. Agencia Española de Medicamentos y Productos Sanitarios. CIMA (Centro de Información de Medicamentos). Ficha técnica de Rubifen® 10 mg. (Spanish Agency of Medicines and Health Products. CIMA ;Drug Information Center). Methylphenidate 10 mg Technical Data Sheet.https://cima.aemps.es/cima/ dochtml/ft/81347/FT_81347.html. Accessed December 14, 2018.
[ Links ]
4. Safer DJ, Zito JM, Fine EM. Increased methylphenidate usage for attention deficit disorder in the 1990s. Pediatrics.1996 Dec;98(6 Pt 1):1084-8.
[ Links ]
5. Narcolepsy summary report. Food and Drug Administration.[Internet] Disponible en:https://www.fda.gov/downloads/forindustry/userfees/prescriptiondruguserfee/ucm402907.pdf (Consultado el 11/12/2018).
[ Links ]
6. Laurito LD, Fontenelle LF, Kahn DA. Hoarding Symptoms Respond to Treatment for Rapid Cycling Bipolar II Disorder. J Psychiatr Pract. 2016 Jan;22(1):50-5.
[ Links ]
7. Corp SA, Gitlin MJ, Altshuler LL. A review of the use of stimulants and stimulant alternatives in treating bipolar depression and major depressive disorder. J Clin Psychiatry. 2014;75(9):1010.
[ Links ]
8. Prada P, Nicastro R, Zimmermann J, Hasler R, Aubry JM, Perroud N. Addition of methylphenidate to intensive dialectical behaviour therapy for patients suffering from comorbid borderline personality disorder and ADHD: a naturalistic study. Atten Defic Hyperact Disord. 2015 Sep;7(3):199-209).
[ Links ]
9. Kaye S, Darke S. The diversion and misuse of pharmaceutical stimulants: what do we know and why should we care? Addiction. 2012 Mar;107(3):467-77.
[ Links ]
10. Padala PR, Dennis RA, Padala KP. Addressing Possible Effects of Sleep Apnea in a Study of Methylphenidate for Apathy in Alzheimer's Disease: Response to Kolla and Mansukhani. Am J Psychiatry. 2018 Aug 1;175(8):792-793.
[ Links ]
11. Mohsin Ali, R. Robert Auger, Nancy L. Slocumb and Timothy I. Morgenthaler. Idiopathic Hypersomnia: Clinical Features and Response to Treatment. J Clin Sleep Med. 2009 Dec 15; 5(6): 562-568.
[ Links ]
12. Parikh MS, Kolevzon A, Hollander E. Psychopharmacology of aggression in children and adolescents with autism: a critical review of efficacy and tolerability. J Child Adolesc Psychopharmacol. 2008;18(2):157.
[ Links ]
13. Husain M and Mehta MA. Cognitive enhancement by drugs in health and disease. Trends Cogn Sci. 2011 Jan;15(1):28-36.
[ Links ]
14. Swanson JM, Volkow ND (2008) Increasing use of stimulants warns of potential abuse. Nature. 2008 May 29;453(7195):586.
[ Links ]
15. Dubljevic V, Ryan C. Cognitive enhancement with methylphenidate and modafinil: conceptual advances and societal implications. 2015 Volume 2015:4 Pages 25-33.
[ Links ]
16. Electronic Health Record (EHR) Impact. Case studies. European Commission. The socio-economic impact of Receta XXI, the regional e Prescribing system of Andalucía’s public health service. European Commission, DG INFSO & Media Julio 2009. [Internet] Disponible en:http://www.juntadeandalucia.es/servicioandaluzdesalud/contenidos/gestioncalidad/diraya/EHRI_case_DIRAYA__final.pdf(Consultado el 12/12/18).
[ Links ]
17. Setlik J, Randall Bond G, Ho M. El abuso de los medicamentos de prescripción para el TDAH por los adolescentes está aumentando al mismo ritmo que las prescripciones de estos medicamentos. Pediatrics (Ed esp). 2009;68(3):123-7.
[ Links ]















