INTRODUCTION
Etoposide is a widely used antineoplastic agent in the treatment of different types of neoplasia such as osteosarcoma, rhabdomyosarcoma, Ewing's sarcoma and some lymphomas and leukemias. Among the adverse events associated with the administration of etoposide there are hypersensitivity reactions in children and adults.
There is conflicting data in the literature regarding incidence of intravenous etoposide hypersensitivity reactions (HR). According to the Summary of products characteristics (SmPC) hypersensitivity occurs in 1-3% of patients1. However, studies describing a much higher incidence, up to 51% of cases can be found in the literature2-4.
Commonly reported symptoms of hypersensitivity reactions include flushing, urticarial rash, mucosal edema, laryngospasm, chest pain, bronchospasm, dyspnea, cyanosis, and hypotension1-2.
The precise mechanism that triggers HR after intravenous etoposide administration is unknown. It has been speculated that it could be related to the excipient polysorbate 80, used as a solvent in the intravenous presentation1. Polysorbate 80 by itself can trigger histamine release, causing typical hypersensitivity symptoms5.
Factors related to etoposide infusion, such as drug concentration (between 0.3 and 1.0 mg / ml), infusion rate (24 mg/m2/h -105 mg/m2/h), and the presence of some excipients, may contribute to hypersensitivity reactions6.
The aim of this study is to assess intravenous etoposide hypersensitivity incidence and to evaluate potential risk factors for hypersensitivity in paediatric patients treated in a third level hospital.
MATERIAL AND METHODS
A retrospective observational study of paediatric patients treated with etoposide since June 2013 to September 2020 was conducted.
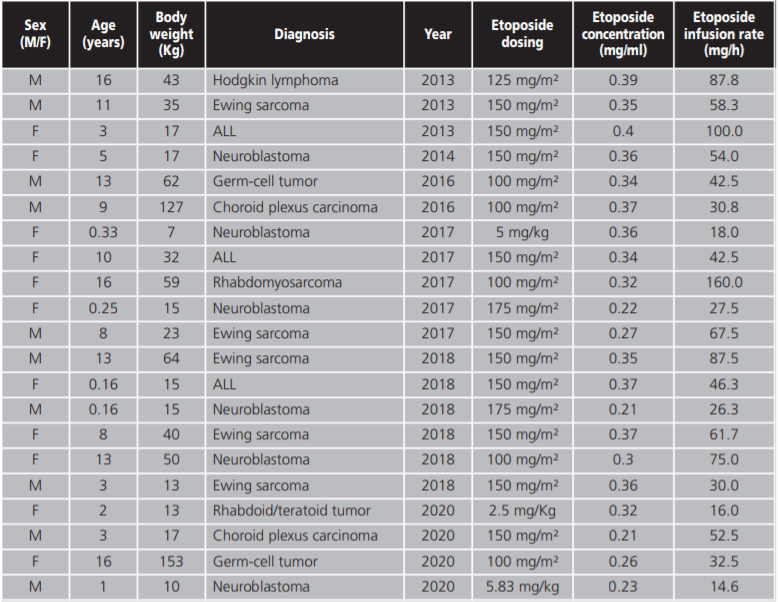
The variables collected were demographics (age, sex), diagnosis, etoposide dose, infusion rate and infusion concentration. Symptoms of hypersensitivity, the grade of hypersensitivity reaction according to the Common Terminology Criteria for adverse Events (CTCAE) and strategies for hypersensitivity reaction management were also collected.
Identified hypersensitivity reactions were reported to the corresponding Pharmacovigilance Center.
Descriptive and univariable correlation analysis between the etoposide HR and the rest of the studied variables were carried out. Statistical analysis was performed by Chi-Square test and logistic regression using IBM SPSS Statistic 24® package. A P <0.05 was considered statistically significant.
RESULTS
A total of 213 patients treated with intravenous etoposide during the period of the study were identified. The median age of the patients was 6.75 years (range 0.16-17 years) and a 58.68% were male.
The indications for etoposide were lymphocytic acute leukaemia 20.18%; neuroblastoma 16.9%; Ewing's Sarcoma 16.9%; Hodgkin's lymphoma 11.27%; myeloid acute leukaemia 8.9% and others 25.82%.
Doses administered ranged from 100-200 mg/m2 and from 2,5-6 mg/kg according to different treatment protocols. The median infusion rate and infusion concentration were 55 mg/h (range 2-200 mg/h) and 0.3 mg/ml (range 0.2-0.5 mg/ml) respectively.
Hypersensitivity reactions occurred in 23 (10.8%) patients, 3 and 20 of them were classified as grade I and grade II reactions of the CTCAE respectively. Symptoms of hypersensitivity reactions were lip cyanosis (n=7), pruritus (n=7), flushing (n=7), nausea (n=5), cutaneous rash (n=5), cough (n=4), rhinoconjunctivitis (n=1), hypotension (n=1), shortness of breath (n=1), abdominal pain (n=1), facial paraesthesia (n=1), fever (n=1) and angioedema (n=1).
All hypersensitivity reactions were successfully managed with medication (corticosteroids and antihistamine). In all cases, subsequent doses were administered with premedication and an infusion rate reduction.
We did not observe any statistical significant association between the variables collected and the apparition of hypersensitivity reactions.
DISCUSSION AND CONCLUSIONS
In this study we report a considerably high incidence of HS, approximately one tenth of patient's, which is significantly higher than the 1-3% incidence reported by the SmPc1. Other studies such as Stockton et al. 20206, Kellie et al. 19914 and Hudson et al. 19933 found also an increased incidence of HR in pediatric patients (27.1%, 33% and 51% respectively), however, the substantially augmented incidence of HR could be explained by high etoposide infusion rates on these studies (200-600 mg/m2/h)6.
Following these results on pediatrics patients, it has been hypothesized that the incidence of pediatric etoposide hypersensitivity may be higher than that of adults, however, this could be explained by the underreporting of mild or moderate reactions, which may contribute to the fact that the data available in the literature on the prevalence of this complication are so disparate, hence the importance of the notification of drug reactions.
Hypersensitivity reactions caused by etoposide can be potentially life-threatening. The rapid selection of an effective treatment is crucial when HR develops. Skipping the offending drug or switching to another drug may decrease the effectiveness of treatment. This is especially relevant in rare diseases, for which there is insufficient evidence on the efficacy of other alternative treatments.
Different strategies have been described for the management of HR to etoposide:
Decrease the infusion rate and/or concentration ofthe preparation along with the administration of premedication with corticosteroids and antihistamines1.
Substitute etoposide for etoposide phosphate, a watersoluble prodrug, which does not contain polysorbate 80 as an excipient in its formulation. However, HR cases have also been reported in patients who have received etoposide phosphate, so the reactions may be due to the active ingredient itself and not only to the excipient2,6. Therefore, patients should have an allergy test previously to rule out that the allergy is due to polysorbate 80 and not to etoposide.
Apply a desensitization protocol with a gradual reintroduction of small doses of etoposide and administering it over long periods of time (4-12 hours) until reaching the therapeutic dose2,7-8.
Use oral etoposide, a formulation of etoposide thatdoes not contain the solvent polysorbate 80 and with which HR has not been described.
In our study all HR were mild, being resolved by standard treatment with antihistamine and corticoids. After an HR occured, the next etoposide infusion rates were decreased alongside the administration of premedication in order to avoid future episodes, these measures were effective on all patients.
As the premedication seems like an effective measure to prevent HR, the possibility of the administration of preparatory medication on all patients who are to receive treatment with etoposide with corticoids and antihistamines has been considered. However, there are some evidence showing that protocols including these medication may mask or delay the appearance of the hypersensitivity reaction3,4,6.
We were unable to establish the variables collected as risk factors for hypersensitivity reactions, probably due to a small sample size derived from the rarity of the event studied. Other studies have observed a relationship between infusion rate and etoposide HR3,4,6.
Limitations of this study include: its retrospective design, its reliability on the documented symptoms and diagnosis of clinical charts and finally, the modest incidence of hypersensitivity reactions limited our ability to compare prevention strategies and establish correlation between these episodes and other studied variables.
CONCLUSIONS
The incidence of hypersensitivity reaction was higher than described in the summary of products information, but lower than described in other studies. All reactions were mild being resolved by standard treatment. We were unable to establish the variables collected as risk factors for hypersensitivity reactions.