INTRODUCTION
Biotechnology, the drug manufacturing strategy that has transformed the healthcare industry over the past 30 years, continues to produce novel, life-saving products. Monoclonal antibodies, biologic response modifiers, and cellular therapies have improved patient outcomes in many debilitating disease-states including hepatitis, cancer, autoimmune and inflammatory diseases. These innovative biologic medications are allowing patients to live longer, healthier lives, and pharmaceutical companies have shifted their focus to the production of these agents to meet the demands of a changing therapeutic landscape.1
Biologic medications are unique in that they are derived from living organisms or contain components of living organisms. The creation of proteins within living entities is complicated and leads to the development of structures that are complex, higher in molecular weight, and much less characterized compared to chemical drug products.2 This makes it virtually impossible to replicate exact structures when manufacturing biologic medications.
Unfortunately, a complicated manufacturing process leads to increased drug development costs.3 As a result, most biologic agents are expensive, which can create a financial burden for governments, insurers, health systems, and patients. In fact, biologic medications can cost upwards of USD 2.1M per treatment.4 Even with the availability of insurance coverage and patient assistance programs, the average individual is unable to afford these agents, resulting in reduced patient access to potentially life-saving medications.5 Considering patients on branded medications are 2-3 times more likely to abandon their treatment than those prescribed generic medications, utilization of a more cost-effective alternative could also lead to better medication adherence.6
Generic medications in the United States generated savings as high as USD 265 billion in 2017 and USD 2 trillion from 2009-2018, while accounting for 90% of all prescription dispenses.6 Biosimilars provide an analogously promising solution to the increased costs associated with biologic agents. They are highly similar versions of reference (or originator) biologics that contain no clinically meaningful differences in terms of safety, purity and potency.7 If successful, biosimilars would increase patient access to more affordable successors to reference biologic medications, potentially saving 1.2 million USD 54 billion over the next ten years.8 However, this is no small feat. The differing nature of chemical drug products and biologic therapies creates distinct challenges that complicate regulatory guidance and adoption of biosimilar medications. As medication experts, pharmacists need to be integral educators, advocates and trailblazers of biosimilar integration into clinical practice across all settings. The objective of this commentary is to make pharmacists across healthcare settings aware of the major practice changing effects and opportunities that the widespread introduction of biosimilar medications hold for future clinical practice.
Barriers to the adoption of biosimilars in clinical practice
In the 1980s, the Hatch-Waxman Act created the foundation toward expediting the approval of generic medications in the United States. Following in its footsteps, regulatory bodies around the world have developed abbreviated drug approval pathways for biosimilar medications.2 However, unlike the generic drug market, manufacturers of biosimilars are tasked with the challenge of developing clinically comparable agents that are similar, but not identical, products. This has resulted in imperfect regulations that govern biosimilar drug approval.
The abbreviated biosimilar application process is slightly different between the European Union and the United States.2 All biosimilar medications undergo a rigorous evaluation of analytical and nonclinical studies to establish high similarity between the structures of the reference and biosimilar product.9 However, biosimilars do not require completion of extensive phase III and phase IV clinical studies.7 This has led regulatory agencies to develop approval pathways that are flexible and subject to individual requirements. While the European Union has opted for a product-specific approval pathway based on biological classification, the United States has adopted a case-by-case approach that takes into account the totality of evidence presented by the manufacturer.2 Though these strategies both address the expected uniqueness of each product, each biosimilar application can likely be different, further confusing defined criteria for approval.
Interchangeability, as it pertains to biologics, is a designation that allows biosimilars to be substituted with reference products without a provider’s approval.7 To date, Europe has not established any regulation on the interchangeability of reference biologic and biosimilar medications.10 European countries typically employ a single-payer healthcare system, which gives them better control over which biologic agents to offer patients without interchangeability being a large hindrance to biosimilar utilizaton.11 In contrast, the United States’ insurance landscape reflects a multi-payer system which prevents universal standardization of biologic medication utilization. Fortunately, the United States Food and Drug Administration (FDA) has finalized its guidance documents on the interchangeability of biosimilar medications which will eventually allow pharmacists to automatically dispense cheaper, therapeutically equivalent alternatives.7 Although no products have been deemed interchangeable as of July 2019, the successful completion of interchangeability clinical trial designs will hopefully be the first step in fulfilling the ultimate vision for biosimilar use in clinical practice in the United States.
Pharmacovigilance programs will also be of great importance concerning biosimilar medications. The lack of extensive clinical evaluations during the approval process has created doubt surrounding long-term efficacy, immunogenicity and adverse event data.2 Though the European Union has established post-marketing monitoring programs for biosimilars, the United States has yet to set defined pharmacovigilance requirements. Without these guidelines, manufacturers do not have the appropriate tools to develop drug monitoring programs. Additionally, limited guidance surrounding post-marketing immunogenicity and adverse event data may hinder the advancement and acceptance of interchangeability designation for biosimilar medications.
Beyond the regulatory barriers facing biosimilar integration into clinical practice, physician knowledge and perception of biosimilar medications has also hindered their uptake in various drug markets. A study conducted by Leonard et al. showed that only 22.9% of providers in the United States and the European Union reported having ‘good’ or ‘complete’ knowledge of biosimilar medications and their appropriate use.12 When asked which concepts were most concerning, providers were apprehensive about differences regarding safety, efficacy, interchangeability and the extrapolation of indications between the biosimilar and reference biologic product.13 This systematic review also showed that, even when providers appeared to be more comfortable with the key concepts of biosimilar medications, they were more likely to prescribe these agents in treatment-naïve patients versus switching patients already on the originator biologic.12
Patient perception and acceptance of biosimilar medications should also be taken into account. Ultimately, they will receive this medication and have the ability to accept or deny administration, depending on how comfortable they are with the product. In a web-based study conducted in the European Union, about 80% of patients on biosimilars understood what they were and that they were used to help reduce drug costs.13 The study further found that patients who were already on biosimilars were confident in their physician’s decision to use a biosimilar therapy compared to when the provider recommended switching from original therapy. This further validates that interchangeability remains a concern for patients as well.
Implications of biosimilars for pharmacy practice
There are over 50 approved biosimilars in drug markets around the world.14 By 2020, nine patents for the top-20 biologic medications are set to expire and pharmaceutical companies have already begun to fill the pipeline with their own biosimilar equivalents. Historically, the United States has lagged behind Europe’s expansion of biosimilar drug options and their uptake into clinical practice. For example, an infliximab biosimilar approved in March 2015 has garnered about 80% market share in the European Union.15 In contrast, Remicade®, the originator infliximab biologic, still holds 96% market share in the United States after the approval of its first biosimilar counterpart in 2016.16 The European Union has shown that successful biosimilar uptake is possible; however, the key to realization starts with overcoming the aforementioned barriers. Pharmacists are uniquely positioned to take a leading role in this effort.
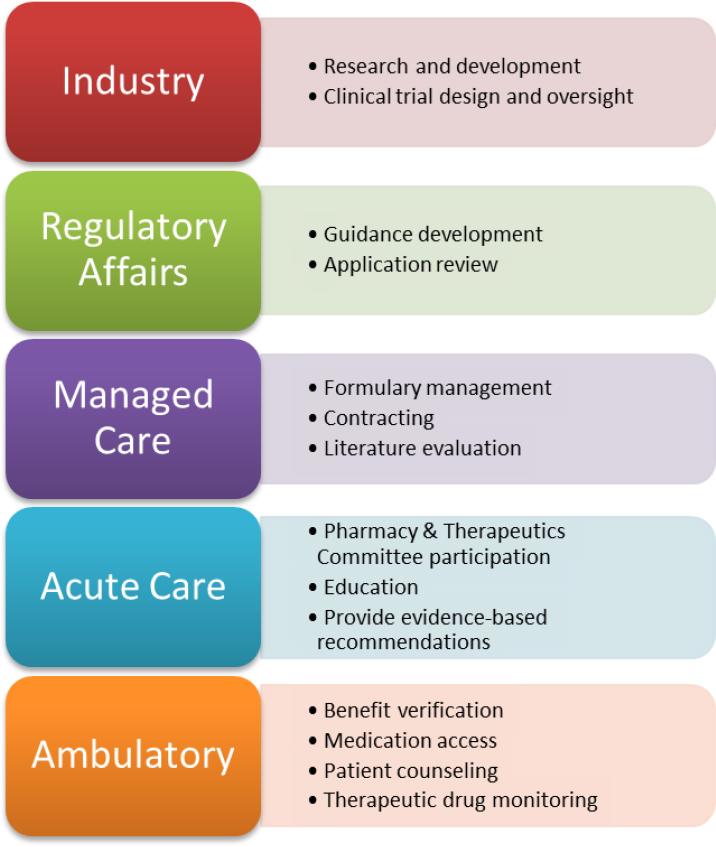
Though the regulatory challenges facing biosimilar medications will need to be addressed at the federal level, pharmacists working all different practice settings can be integral catalysts for change. The FDA and other regulatory agencies frequently ask for feedback through adverse event reporting databases and other comment forums to collect biosimilar safety data and improve clinical practice. Pharmacists can submit research or case studies involving interchangeability, pharmacovigilance programs or other experiences to provide lawmakers with real-world information to help develop guidance documents. Additionally, industry and regulatory affairs pharmacists can be organizers of improved biosimilar evaluation. Pharmacists working for pharmaceutical companies will need to oversee the development of clinical trials in order to ensure that manufacturers are producing safe and effective agents. Regulatory affairs pharmacists may then be involved in biosimilar application review, which involves clinical judgement when reviewing the totality of evidence presented by the manufacturer. This is an area where pharmacists can take a leadership role as they are trained in biopharmaceutics and literature evaluation.
While the ongoing discussion surrounding interchangeability designation has hindered biosimilar adoption in the United States, managed care pharmacists are preferentially positioned to act as facilitators for the introduction of biosimilars into clinical practice. Companies may determine a biosimilar is preferred over its original biologic counterpart on an institution’s formulary and this will require patients to utilize the biosimilar option in order for the therapy to be covered. This is a key strategy to promote the acceptability of biosimilars while regulatory bodies determine the appropriateness of interchangeability between reference biologics and biosimilar medications. Drug manufacturers of biologic agents are working diligently to create financially attractive contracts with managed care companies to designate biosimilars as the preferred therapeutic option. Pharmacists working in this setting will review safety, efficacy and immunogenicity studies to ensure the proper utilization of biosimilar medications. As key members of pharmacy and therapeutics committees, pharmacists can provide leadership to determine the appropriate restrictions or prior authorizations required for each biosimilar that is considered for addition to their plan’s drug formulary. They may also be participating in patient monitoring programs to gather long-term safety data of biosimilar medications.
Once a biosimilar has reached the market, pharmacists working in patient care will need to be aware of the practical, day-to-day handling of biosimilar medications. In the acute care setting, biosimilar utilization will be primarily driven by formulary status. Pharmacists, physicians and other key stakeholders will have the opportunity to decide on a biologic agent of choice to utilize throughout their hospital or health system for each molecule. It will be important to be aware of key biosimilar concepts in order to be a productive member of the team. By developing policies and procedures to prepare for the increasing biosimilar drug market, the integration of biosimilars into the hospital setting can be done in a fairly controlled manner. Nonetheless, hospital pharmacists may encounter biosimilars that will be utilized on a daily basis. Recent reports have stated that the first insulin biosimilar could be available as early as the end of 2019.17
Ambulatory pharmacists will likely encounter unique, expensive therapies used to treat Crohn’s disease, cancer and other inflammatory or rheumatoid diseases that significantly impact patient morbidity and mortality. As frontline members of the healthcare system, pharmacists will likely be heavily involved in education, benefit verification and medication access. Often, only one biologic agent is covered by an insurance plan and using an alternative agent likely would require a step therapy approach or could result in a claim denial. Ensuring that the patient receives a biosimilar that is appropriate and aligns with their insurance company’s formulary will be essential to guaranteeing access and affordability. Furthermore, many insurance companies require extensive paperwork and follow-up to approve a prescribed biologic medication. Pharmacists may need to consistently engage with patients to confirm adherence to treatment regimens so patients may continue to receive benefits or patient assistance. Additionally, some pharmacists may be involved in therapeutic drug monitoring, evaluating patients for efficacy or signs of immunogenicity or drug toxicity.
Interchangeability status will be of great importance in the ambulatory care setting as well. The creation of an interchangeable class of biosimilar medications will allow for the automatic switching of biosimilar products at the pharmacy level, mirroring the generic drug market. Pharmacists will need to become familiar with the Purple Book, which is a list of licensed biological products (including biosimilars and reference biologic agents) approved for interchangeability under the Public Health Service Act.18 It is important to keep in mind that each state may possess different laws governing the interchangeability of biologic medications.19 Inappropriately substituting a reference biologic for a non-interchangeable biosimilar without the provider’s approval could be prohibited and lead to action taken against a pharmacy’s or pharmacist’s license. It would behoove pharmacy informatics teams to build logic into electronic health records or retail pharmacy databases that would prevent inappropriate interchanging from happening.
Ultimately, pharmacists working in all settings will need to be educators and advocates for biosimilar introduction into clinical practice (Figure 1). Understanding of the nature of reference biologics and how they relate to their biosimilar counterparts is still challenging for patients, providers and other healthcare practitioners. Therefore, it is important that pharmacists host continuing education programs, in-services or other presentations to explain the basic principles of biosimilar medications and increase confidence in their utility. More than anything, pharmacists will be a resource to answer questions regarding the appropriate use of biosimilars, and it is important that individuals are knowledgeable enough to provide sufficient recommendations.
Future direction
The time is now for pharmacists to get involved with biosimilar introduction into clinical practice. Regulatory and practical barriers have created a slow integration in the United States, and a recent publication posited that the manufacturing of biosimilars could phase out as drug companies become disinterested in the process.20 The education of pharmacists, physicians, nurses, patients and other key stakeholders will be the most important factor to biosimilar acceptance. Better adoption in clinical practice will occur as clinicians, patients and other stakeholders increase their understanding of the role of biosimilars in patient care. Though the path to biosimilar uptake can be difficult to navigate, pharmacists are positioned to take a lead. If pharmacists fail to prepare and adapt to the changes biosimilars are expected to bring, it may lead to a lost opportunity for the profession to improve patient care.