My SciELO
Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista de Osteoporosis y Metabolismo Mineral
On-line version ISSN 2173-2345Print version ISSN 1889-836X
Rev Osteoporos Metab Miner vol.8 n.4 Madrid Oct./Dec. 2016
ORIGINAL
Functional study of promoter gene polymorphisms of sclerostin
Estudio funcional de los polimorfismos del promotor del gen de la esclerostina
Pérez-Campo F.M.2, Sañudo C.1, Krebesova R.1, Delgado-Calle J.3 and Riancho J.A.1
1 Departamento de Medicina Interna - Hospital U.M. Valdecilla - Universidad de Cantabria - IDIVAL - Santander (España)
2 Departamento de Biología Molecular - Universidad de Cantabria - Santander (España)
3 Departamento de Anatomía y Biología Celular - Facultad de Medicina de la Universidad de Indiana - Centro Médico de Administración de Veteranos Roudebush - Indianápolis - Indiana (EE.UU.)
This research was carried out with the support of a grant from the FEIOMM 2013 "Interaction between Osterix, Runx2 and sclerostin: molecular mechanisms and impact on bone mass" and the Institute of Health Carlos III (PI12-615), co-financed with the European Union's FEDER funds.
Work rewarded with the scholarship of Molecular Bone Biology FEIOMM 2013.
SUMMARY
Sclerostin, encoded by the SOST gene, inhibits the Wnt pathway and, consequently, tends to decrease bone mass. Some polymorphisms of the SOST promoter have been associated with bone mineral density (BMD), but the molecular mechanisms involved are unknown. The aim of this study was to study the functional role of one polymorphism in vitro. We cloned the proximal promoter region of SOST gene, containing different alleles at the rs851054 SNP, in luciferase reporter vectors and transfected them into the cell lines HEK-293T, SAOS-2 and HOS-TE85. We did not find significant differences in the transcriptional activity of vectors with either the A or the G allele of the SNP. The co-transfection of vectors expressing RUNX2 and OSX markedly increased the transcriptional activity of the SOST promoter constructs (A allele, 2.5±0.9 fold, p<0.05; G allele, 1.9±0.8 fold, p<0.05), without significant differences between the rs851054 alleles. Moreover, no allele differences were detected in EMSAs.
In conclusion, the DNA region upstream of the TSS of the SOST gene has a strong promoter activity that is enhanced by RUNX2 and OSX. Frequent allelic variants in this region have been associated with BMD, but the mechanisms involved remain to be elucidated because no functional differences between alleles were detected in vitro.
Key words: sclerostin, gene regulation, polymorphisms, transfection.
RESUMEN
La esclerostina, codificada por el gen SOST, es un inhibidor de la vía Wnt, y por ello tiene una influencia negativa sobre la masa ósea. Algunos polimorfismos del promotor de SOST se han asociado a diferencias en la densidad mineral ósea (DMO) en varios estudios, pero se desconocen cuáles son los mecanismos moleculares implicados. El objetivo de este estudio fue comprobar las consecuencias funcionales de uno de esos polimorfismos in vitro. Para ello se clonó la región promotora proximal del gen SOST, con diferentes alelos del polimorfismo rs851054, y se transfectaron en células HEK-293T, SAOS-2 y HOS-TE85. En ningún caso se observaron diferencias significativas en la actividad transcripcional entre los vectores con el alelo A y los vectores con el alelo G. La co-transfección de vectores de expresión de los factores de transcripción RUNX2 y OSX estimuló claramente la actividad transcripcional (2,5±0,9 veces sobre el valor basal para el alelo A y 1,9±0,8 veces para el alelo G; en ambos casos, p<0,05), sin que hubiera, sin embargo, diferencias entre los alelos. Tampoco se hallaron diferencias en la fijación a proteínas nucleares analizadas en experimentos de retardo de la movilidad electroforética.
En conclusión, la región situada antes del inicio de la traducción del gen SOST tiene una potente actividad promotora, que es aumentada por los factores RUNX2 y OSX. Las variantes frecuentes de esta región se han asociado con la DMO, pero los mecanismos implicados son aún desconocidos, puesto que los alelos analizados no muestran diferencias en la actividad transcripcional in vitro.
Palabras clave: esclerostina, regulación génica, polimorfismos, transfección.
Introduction
Long-term bone mass development is determined by the balance between resorption and bone formation. So, when bone forming activity is insufficient to replace the part destroyed during resorption, it will inevitably lead to decreased bone tissue, characteristic of osteoporosis. Osteoblasts, responsible for bone formation, derived from mesenchymal stem cells, can also lead to other cell types such as chondrocytes or adipocytes. The proliferation and differentiation of osteoblast precursors are controlled by different intracrine, paracrine and endocrine factors. Thus, some transcription factors, such as RUNX2 and Osterix (OSX) are considered essential in the early stages of osteoblast differentiation. The Wnt pathway also plays an important role1. Wnt ligands join the cell membrane receptors together and, through various intracellular mediators, modify the expression of target genes that generally promote bone formation and increasing osteoprotegerin (OPG) expression, which secondarily is a decrease in resorption. Wnt receptor ligands are molecular complexes which include at least two proteins: Frizzled and LRP, of which there are several forms2,3. As with many other regulatory systems, there are also inhibitors of the Wnt pathway. One of the most widely studied is the sclerostin, encoded by the SOST gene. This gene is expressed preferentially in the osteocytes4-8. Being an inhibitor of the Wnt pathway, sclerostin has a negative influence on bone mass. Its role in skeletal biology seems important. Indeed, neutralizing sclerostin antibodies exert a potent anabolic effect on the skeleton, and the SOST gene inactivating mutations cause an exaggerated increase in bone mass9-11.
In line with the biological role of sclerostin, allelic variants of the SOST gene appear to influence bone mass. Thus, in some studies genome scan (GWAS) and other genetic association studies, we found a link between some common SOST gene polymorphisms and bone mineral density (BMD). Our group also found an association between some polymorphisms located in the promoter region of SOST and BMD in postmenopausal women12. The aim of this study was to explore the regulatory capacity of one of these polymorphisms with functional in vitro studies. we used vectors in which the promoter of SOST is inserted in front of a reporter gene encoding a protein (luciferase) whose activity can be easily measured. Experiments were carried out to test the binding capacity of nuclear proteins to these regions assessing delay electrophoretic mobility.
Material and methods
Construction of reporter vectors
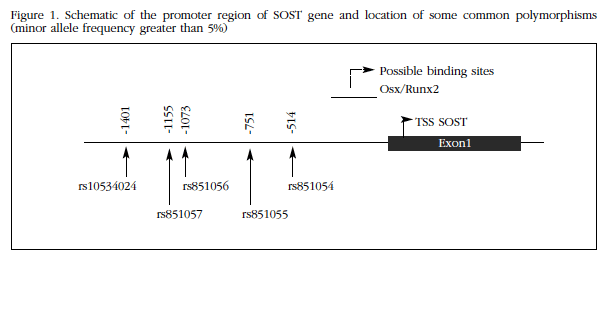
SOST promoter (1-1440) was cloned from genomic DNA of two individuals with known genotype homozygous for alleles A and G, respectively, of the rs851054 polymorphism (Figure 1). Both fragments were otherwise identical and equal to the reference of the human genome sequences. DNA extraction occurred after informed consent, within a study of genetic factors involved in osteoporosis, authorized by the Ethics Committee on Clinical Research. For amplification of the corresponding genomic fragments, PCR primers designed with the Primer3 program were used which included sequences for the restriction enzymes Smal and Xhol, to facilitate further cloning. PGL2 vector and amplicon were cut with these enzymes and the fragments were ligated using T4 DNA ligase (NE Biolabs). The vectors were transfected into DH5α competent cells (Invitrogen), which were then grown on agar plates and ampicillin media. Selected colonies were expanded in liquid culture, and then plasmids were extracted and found to contain the correct inserts without mutations, by conventional sequencing. Therefore, in these vectors SOST promoter sequence was found driving transcription of the luciferase-encoding reporter gene. Thus the luciferase activity measurement reflected transcription promoter activity of the SOST allele sequence which was cloned into the vector.
Transfection and analysis of transcriptional activity
HEK-293T cells (a line derived from human kidney) and human osteoblastic lines Saos-2 and HOS-TE85 were transfected with vectors containing the promoter sequences of SOST and a RSV-βGAL control vector, constitutively expressing the LacZ gene, encoding β-galactosidase, in order to normalize the results which depend on transfection efficiency. As a negative control and parallel transfection with empty vector was also performed. For transfection HEK-293T cells 125,000 (50,000 or Saos-2 or HOS-TE85) were seeded into each well of a 24 well plate. After reaching 80% confluency were transfected 500ng vector using Lipofectamine 3000 according to the manufacturer's recommendations (Invitrogen). At 48 hours, the medium was aspirated and the cells with 70 µl buffer, after which galactosidase activity (Galacto-Light PlusTM β-Galactosidase Reporter Gene Assay System, Applied Biosystems) was measured and luciferase (Luciferase lysed Assay System, Promega) by luminometry. The co-transfection with expression vectors and OSX13 RUNX2 was performed following a similar procedure, but ensuring that the total amount of exogenous DNA to be transfected was held constant in all wells trays.
From each transfection, double and triple techniques were performed. Furthermore, each experiment was repeated at least three different times to obtain biological triplicates. The results were expressed as the ratio between luciferase and galactosidase activity in cell lysates.
Analysis of nuclear protein binding
Protein extracts were obtained from 50 x 106 HOS-TE85 cells. To do this, in a lysis buffer was used containing protease inhibitors (50 mM KCl, 0.5% NP-40, 25 mM HEPES, 1.5 pM leupeptin, aprotinin 46.88 µM, 125 µM DTT, 1 mM PMSF). These were centrifuged for 1 minute at 10,000 rpm at 4oC. After washing, the cells were re-suspended in 100 µl of extraction buffer (500 mM KCl, 25 mM HEPES, 10% glycerol, 1.5 pM leupeptin, aprotinin 46.88 µM, 125 µM DTT, 1 mM PMSF) and centrifuged for 5 minutes at 14,000 rpm at 4oC. The supernatant was recovered, the protein concentration was quantified and adjusted to 5 µg/µl.
To test the delay of electrophoretic mobility shift assay (EMSA), oligonucleotide pairs were used, including IR dye labeling 700 in the 5 'end of the "forward" component of each pair (Biolegio). The sequences were as follows:
rs851054 allele A Fwd: AACAGAAACACCTTGGGCCA
rs851054 allele A Rev: TGGCCCAAGGCGTTTCTGTT
rs851054 allele G Fwd: AACAGAAACGCCTTGGGCCA
rs851054 allele G Rev: TGGCCCAAGGCGTTTCTGTT
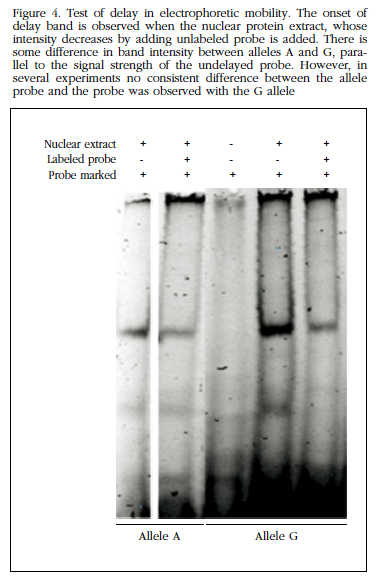
Prefabricated gels 6% polyacrylamide (Invitrogen) and the Odyssey Infrared EMSA kit were used for retardation. After annealing, the oligonucleotides were incubated with protein extract for 20 minutes, loaded on the gel and were run at 70 V for 60 minutes, after which the images of the gels were captured. In these experiments, if the probes bind nuclear proteins occurs a delay in electrophoretic mobility compared to that experienced by the isolated probe migration.
Results
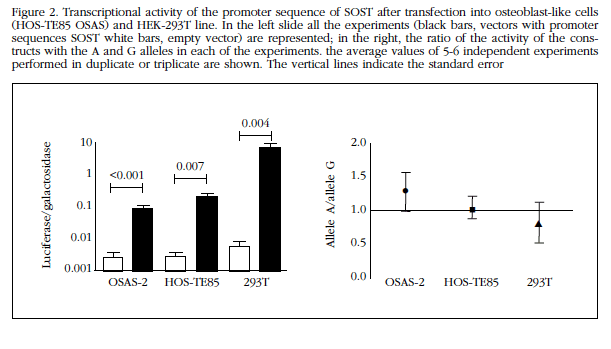
Constructions SOST promoter sequence showed high transcription activating capacity by increasing levels of luciferase expression up to 1,000 times compared with empty vectors. The activity was apparently higher in HEK-293T cells than in the other lines (Figure 2).
However, no significant differences between the two alleles SOST promoter in any of the cell lines tested. In fact, the ratio between the transcriptional activity of alleles A and G (Ratios A/G) in Saos-2 and HOS TE85-293T cells were 1.3±0.7, 1.0±0.4 and 0.8±0.7, respectively. None of the three was significantly different from the unit (Figure 2).
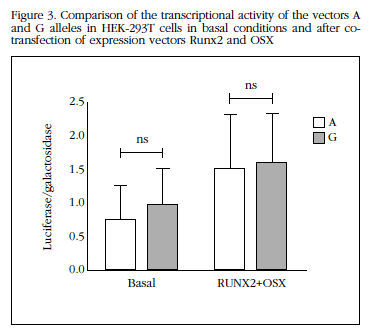
The co-transfection of vectors expressing constitutively RUNX2 and OSX increased transcriptional activity of the SOST promoter (on average 2.5±0.9 times above baseline for allele A and 1.9±0.8 times to the G allele; both p<0.05). This increase was also be independent of which allele was present in the SOST promoter (Figure 3).
Furthermore, in experiments delay electrophoretic mobility see that the region where the polymorphism lies was able to bind nuclear proteins, presumably with regulatory function without us to observe differences between alleles (Figure 4). Therefore, no further studies were performed to identify the nature of these proteins.
Discussion
The role of sclerostin in bone biology is undeniable, as revealed by the sharp BMD increase observed after administration of romosozumab, a monoclonal antibody that blocks sclerostin action, and excessive bone mass experienced by patients with sclerosteosis or Van Buchem syndrome or carriers of rare mutations that induce a rare disease such as loss of SOST gene function14. Although the evidence is limited, several studies suggest that frequent allelic variants of the gene can also influence, to a lesser extent, BMD and osteoporotic fracture risk. In fact, in a study of genomic sweep with a large sample size of rs4792909 polymorphism association was found with BMD15. This polymorphism is located in an intergenic region, being the closest SOST gene, located at about 40 kb. On the other hand, several groups, including ours, have analyzed the relationship of some common polymorphisms in the promoter region of SOST with BMD. So, we found a significant association of rs851054 and rs851056 polymorphisms, only separated by 560 bp and members of the same haplotype block with BMD of the spine in women12. Some authors have confirmed the association of the polymorphism in the SOST promoter with BMD in other population groups 16-20. On the other hand, in a recent study involving a small group of patients, an association of rs851054 alleles with the expression of SOST in bone tissue was found21.
The main objective of this study was to explore the potential functional impact of one of these polymorphisms and to analyze their influence on the transcriptional activity of the SOST gene promoter. Transfection experiments with reporter vectors and delay in electrophoretic mobility did not reveal differences between the allelic variants studied. There are several limitations of the models used and numerous reasons that could explain these negative results. First, it could be argued that the in vitro model does not adequately reflect the in vivo situation. For example, it cannot be excluded that the regulatory activity requires genomic regions with activity enhancer, promoter and far not included therefore in the region cloned into the vectors. On the other hand, at least theoretically it might be thought that the difference in allele activity is only expressed in response to regulatory factors in certain cell types such as osteocytes, but are absent in the cell types used in our experiments. Second, it appears that the association with BMD might rely on other common polymorphisms in linkage disequilibrium with those studied here. If so, they should be in distant, remote regions of the promoter, since in this region there is a strong linkage. In this regard, it should be noted that there is a regulatory region of SOST located at about 50 kb, containing some polymorphisms linked inconsistently with BMD20,22. This region is capable of fixing MEF2C transcription factor and plays an important role in regulating SOST because their lack causes the Van Buchem disease phenotype in humans and increased bone mass in murine models23,24. A third possibility is that the association with BMD depends on some low frequency polymorphisms located in the promoter region. Finally, polymorphisms might act through epigenetic mediators that cannot be adequately recapitulated in in vitro models. Further studies are thus required including the systematic analysis of the variants of the 5' and 3' of the gene and others which are associated with considering the tridimensional structure of chromatin and mutagenesis studies in vivo to clarify the mechanisms by which these allelic variants may modulate the activity of sclerostin and associate with differences in bone mass25.
Our study confirmed previous results indicating that RUNX2 and OSX increase the expression of SOST in humans13. This indicates that these transcription factors have a complex role in the osteoblastic line. On the one hand, they have a well-established role as determinants of the early stages of differentiation of mesenchymal cells into osteoblasts and enable an adequate number of bone-forming osteoblasts. They also promote SOST promoter activity. Therefore, in cells capable of expressing this gene, such as osteocytes, the secretion of sclerostin could promote and thus help prevent exaggerated bone formation. In any case, the SOST promoter variants do not appear to influence the response to these factors.
In conclusion, in this study we have confirmed that the region located before the start of translation of the gene SOST has potent promoter activity, which also induced transcription factors RUNX2 and OSX. Frequent variants of this region have been associated with bone mass, but the mechanisms involved are still unknown, since the alleles show no differences in in vitro transcriptional activity.
Acknowledgements
The authors would also like to express their grateful to Dr. Svante Paabo for generously supplying Runx2 expression plasmid.
Declaration of interest
The authors declare no conflicts of interest.
![]() Correspondence:
Correspondence:
José A. Riancho
Departamento de Medicina Interna
Hospital U.M. Valdecilla
Avda. Valdecilla, sn
39008 Santander (España)
E-mail: rianchoj@unican.es
Date of receipt: 16/07/2016
Date of acceptance: 25/09/2016
Bibliography
1. Riancho JA, Hernandez JL. Pharmacogenomics of osteoporosis: a pathway approach. Pharmacogenomics. 2012;13(7):815-29. [ Links ]
2. Baron R, Rawadi G. Wnt signaling and the regulation of bone mass. Curr Osteoporos Rep. 2007;5:73-80. [ Links ]
3. Bodine PV, Komm BS. Wnt signaling and osteoblastogenesis. Rev Endocr Metab Disord. 2006;7:33-9. [ Links ]
4. Semenov M, Tamai K, He X. SOST is a ligand for LRP5/LRP6 and a Wnt signaling inhibitor. J Biol Chem. 2005;280(29):26770-5. [ Links ]
5. Li X, Zhang Y, Kang H, Liu W, Liu P, Zhang J, et al. Sclerostin binds to LRP5/6 and antagonizes canonical Wnt signaling. J Biol Chem. 2005;280:19883-7. [ Links ]
6. Moester MJ, Papapoulos SE, Lowik CW, van Bezooijen RL. Sclerostin: current knowledge and future perspectives. Calcif Tissue Int. 2010;87:99-107. [ Links ]
7. Ott SM. Sclerostin and Wnt signaling--the pathway to bone strength. J Clin Endocrinol Metab. 2005;90:6741-3. [ Links ]
8. Poole KE, van Bezooijen RL, Loveridge N, Hamersma H, Papapoulos SE, Lowik CW, et al. Sclerostin is a delayed secreted product of osteocytes that inhibits bone formation. FASEB J. 2005;19:1842-4. [ Links ]
9. Loots GG, Kneissel M, Keller H, Baptist M, Chang J, Collette NM, et al. Genomic deletion of a long-range bone enhancer misregulates sclerostin in Van Buchem disease. Genome Res. 2005;15:928-35. [ Links ]
10. Balemans W, Van Hul W. Identification of the disease-causing gene in sclerosteosis--discovery of a novel bone anabolic target? J Musculoskelet Neuronal Interact. 2004;4:139-42. [ Links ]
11. Kim CA, Honjo R, Bertola D, Albano L, Oliveira L, Jales S, et al. A known SOST gene mutation causes sclerosteosis in a familial and an isolated case from Brazilian origin. Genet Test. 2008;12:475-9. [ Links ]
12. Valero C, Zarrabeitia MT, Hernandez JL, Pineda B, Cano A, Garcia-Perez MA, et al. Relationship of sclerostin and secreted frizzled protein polymorphisms with bone mineral density: an association study with replication in postmenopausal women. Menopause. 2011;18:802-7. [ Links ]
13. Perez-Campo FM, Santurtun A, Garcia-Ibarbia C, Pascual MA, Valero C, Garces C, et al. Osterix and RUNX2 are Transcriptional Regulators of Sclerostin in Human Bone. Calcif Tissue Int. 2016;99(3):302-9. [ Links ]
14. Balemans W, Van Hul W. Human genetics of SOST. J Musculoskelet Neuronal Interact. 2006;6:355-6. [ Links ]
15. Estrada K, Styrkarsdottir U, Evangelou E, Hsu YH, Duncan EL, Ntzani EE, et al. Genome-wide meta-analysis identifies 56 bone mineral density loci and reveals 14 loci associated with risk of fracture. Nat Genet. 2012;44:491-501. [ Links ]
16. Kuipers AL, Zhang Y, Yu S, Kammerer CM, Nestlerode CS, Chu Y, et al. Relative influence of heritability, environment and genetics on serum sclerostin. Osteoporos Int. 2014;25(3):905-12. [ Links ]
17. Sims AM, Shephard N, Carter K, Doan T, Dowling A, Duncan EL, et al. Genetic analyses in a sample of individuals with high or low bone density demonstrates association with multiple Wnt pathway genes. J Bone Miner Res. 2008;23:499-506. [ Links ]
18. Yerges LM, Klei L, Cauley JA, Roeder K, Kammerer CM, Moffett SP, et al. High-density association study of 383 candidate genes for volumetric BMD at the femoral neck and lumbar spine among older men. J Bone Miner Res. 2009;24:2039-49. [ Links ]
19. Mencej-Bedrac S, Prezelj J, Kocjan T, Komadina R, Marc J. Analysis of association of LRP5, LRP6, SOST, DKK1, and CTNNB1 genes with bone mineral density in a Slovenian population. Calcif Tissue Int. 2009;85:501-6. [ Links ]
20. Uitterlinden AG, Arp PP, Paeper BW, Charmley P, Proll S, Rivadeneira F, et al. Polymorphisms in the sclerosteosis/van Buchem disease gene (SOST) region are associated with bone-mineral density in elderly whites. Am J Hum Genet. 2004;75:1032-45. [ Links ]
21. Lhaneche L, Hald JD, Domingues A, Hannouche D, Delepine M, Zelenika D, et al. Variations of SOST mRNA expression in human bone are associated with DNA polymorphism and DNA methylation in the SOST gene. Bone. 2016;92:107-15. [ Links ]
22. Balemans W, Foernzler D, Parsons C, Ebeling M, Thompson A, Reid DM, et al. Lack of association between the SOST gene and bone mineral density in perimenopausal women: analysis of five polymorphisms. Bone. 2002;31:515-9. [ Links ]
23. Collette NM, Genetos DC, Economides AN, Xie L, Shahnazari M, Yao W, et al. Targeted deletion of Sost distal enhancer increases bone formation and bone mass. Proc Natl Acad Sci. U S A 2012;109(35):14092-7. [ Links ]
24. Balemans W, Cleiren E, Siebers U, Horst J, Van Hul W. A generalized skeletal hyperostosis in two siblings caused by a novel mutation in the SOST gene. Bone. 2005;36:943-7. [ Links ]
25. Huang Q. Genetic study of complex diseases in the post-GWAS era. J Genet Genomics. 2015;42(3):87-98. [ Links ]











 text in
text in