My SciELO
Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista de Osteoporosis y Metabolismo Mineral
On-line version ISSN 2173-2345Print version ISSN 1889-836X
Rev Osteoporos Metab Miner vol.9 n.1 Madrid Jan./Mar. 2017
https://dx.doi.org/10.4321/s1889-836x2017000100007
Revisión
Osteoporosis in rheumatic diseases and glucocorticoid induced
1Universidad de Especialidades Espíritu Santo - Samborondón (Ecuador)
2Hospital Cosme Argerich - Buenos Aires (Argentina)
3Hospital Luis Vernaza. Guayaquil (Ecuador)
4Centro de Reumatología y Rehabilitación - Guayaquil (Ecuador)
Introduction
Osteoporosis is a systemic skeletal disease characterized by decreased bone mineral density with alterations in bone microarchitecture and increased risk of fracture, involving genetic and environmental factors. It is classified as primary when it depends on physiological processes, as in the case of menopause and normal aging, and secondary when conditioned by other pathologies or in relation to medications, as in osteoporosis secondary to rheumatic diseases and glucocorticoid-induced osteoporosis (GIO).
The current trend of widespread corticoid use has led to GIO being the most common cause of drug-induced osteoporosis, constituting a worldwide problem. Approximately 0.5% of the general population and 1.7% of women over 55 years of age receive oral glucocorticoids and, despite the existence of diagnostic methods and adequate measures to prevent GIO, less than 14% receive some type of treatment to avoid loss of bone mass due to glucocorticoids [1,2,3].
In patients with rheumatic diseases such as rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), ankylosing spondylitis (AS), polymyalgia rheumatica (PMR), and vasculitis, among others, osteoporosis is an associated comorbidity. This may be due to the use of glucocorticoids over long periods of time as part of the accepted therapy of these diseases, or it may also be associated with the inflammatory activity of the condition and its impact on the bone. In any case, these patients are more likely to suffer fractures due to fragility, which leads to a decrease in the quality of life [3,4].
The clinical relevance of osteoporosis in rheumatic diseases is underestimated. Studies have shown that patients with RA receiving oral glucocorticoids who underwent a control bone densitometry had osteopenia/osteoporosis in 23% of the cases, of which 42% were prescribed at least one medication to reduce bone loss [3].
This indicates an insufficient appreciation of the clinical problem and a lack of consensus on osteoporosis detection and treatment in rheumatic diseases.
Effects of inflammation on bone turnover
The determinants of inflammation on the bone are proinflammatory cytokines. Thus, the alpha (TNF- α) tumor necrosis factor, interleukins (IL) 1 and 6 (IL-1 and IL-6) favor bone resorption by directly or indirectly promoting osteoclastogenesis. IL-17 enhances activator receptor ligand expression for nuclear factor kappa B (RANK-L), which in turn is a key part of the bone resorption process (Figure 1).
TNF acts on proinflammatory signaling pathways within the joints, influencing bone turnover, at the same time as it seems to be involved in developing bone erosions and the progression of osteoporosis. In addition, TNF has been shown to promote pre-osteoclastic cell formation expressing the activator receptor for nuclear factor kappa B (RANK), promoting overexpression of RANK-L osteo-protegerin (OPG) and inhibiting the maturation and function of the osteoblasts, because it increases the expression of the synovial fibroblasts [5].
The relationship between proinflammatory cytokines and resorption is well established. However, there is a lack of evidence on the relationship between cytokines and bone formation. IL-4, IL-12, IL-8 and interferon are known to promote bone formation due to increased OPG/RANKL ratio, thus inhibiting osteoclastogenesis [6].
On the other hand, the osteoblast produces cytokines and in its immature state promotes the formation of RANK-L and in its mature state the production of OPG, showing that the osteoblast maturation process is essential to balance the relationship between the inflammatory process and bone mass [6].
Rifas and Weitzmann discovered a cytokine and called it a secreted osteoclastogenic factor of activated T cells (SOFAT)and demonstrated that it induces the osteoblastic production of IL-6 and the formation of osteoclasts in the absence of osteoblasts or RANKL, and that it is insensitive to OPG effects. This demonstrates that SOFAT is a potent inducer of IL-6 production, playing a key role in the local inflammatory response, and may indirectly exacerbate bone destruction in rheumatoid arthritis through multiple IL-6 mediated processes [7].
Acute and chronic inflammatory processes in rheumatic diseases cause bone damage because inflammation increases the number and activity of osteoclasts and, at the same time, the activation of T cells. During infectious processes increases RANKL production, which, in turn, stimulates osteoclastogenesis. It has been demonstrated that the application of OPG can reverse this effect in diseases such as RA and periodontitis [8].
Osteoporosis in specific rheumatic diseases
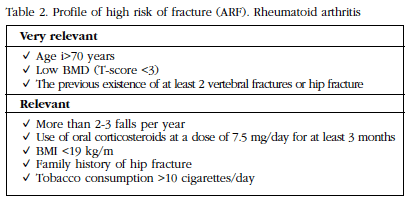
It is known that subjects with a history of chronic autoimmune diseases have a higher risk of developing osteoporosis due to different causes, such as specific alterations that affect bone metabolism, induce loss and sometimes inhibit formation, or to meet factors such as: sex (predominance in women), low physical activity, underlying disease, smoking, low body mass index (BMI), vitamin D deficiency, etc. (Table 1).
The Multidisciplinary Forum on Management of Patients with High Risk of Osteoporotic Fracture (HRF) has suggested classifying risk factors as follows [9]:
- Keys: age over 70 years, history of previous frail fracture (vertebral or hip), glucocorticoid intake (7.5 mg/day for 3 months or more) and BMD (T-score ≤3).
- Important: maternal history of hip fracture, BMI ≤20, frequent falls in the elderly, low measurements in activity and physical function.
- Moderate: levels of 25(OH)-vitamin D ≤30 ng/dl, some lifestyle-related harmful factors (smoking, excessive alcohol intake, sedentary lifestyle or excessive coffee consumption).
Rheumatoid arthritis (RA)
RA is an autoimmune inflammatory disease of unknown origin that is more common in young women, between the third and fifth decades of life [4]. It is a deforming and disabling disease that directly affects the joint causing bone erosions, which end up injuring the joint and resulting in its deformity.
Osteoporosis is a common complication of RA and is classified as follows [10]:
- Yuxta-articular: early and secondary to local factors.
- Generalized: late and multi-causal in origin (rest, drugs, disease activity, degree of functional alteration and deterioration of muscle mass).
The frequency of osteoporosis in RA is variable. Sinigaglia et al reported an incidence of osteoporosis of 10-56% in patients with RA [3]. Forsblad added that this range will depend on the population studied, and stressed the importance of joint damage in RA and generalized osteoporosis [11].
Patients with RA have a higher risk of fracture compared to the general population. A retrospective study of 30,262 patients showed a higher risk of hip fracture (RR=2.0) and spine (RR=2.4) vs the control group, and the risk increased due to prolonged use of corticosteroids (RR=3.4) [12].
The high risk of fracture in RA patients has also been associated with disease-specific factors [13] (Table 2).
Corticosteroid use is an independent predictor of the decrease in bone mass at the lumbar and femoral levels. A low dose steroid regimen (5 mg prednisone) is associated with a 50% increase in the risk of osteoporosis [14]. This demonstrates that treatment with oral corticosteroids of more than 5 mg of prednisone is able to reduce bone mass with a rapid increase in the risk of fracture during the treatment period [15,16]. Taking into account this risk factor, an American College of Rheumatology (ACR) committee recommends screening for osteoporosis using BMD and initiating treatment in patients with T-score ≤1.0, preferably with bisphosphonates and calcium supplements and Vitamin D. These patients should be monitored annually and treatment should continue while receiving glucocorticoids [17].
Longitudinal and cross-sectional studies have shown that disease modifying anti-rheumatic drugs (DMARDs), such as methotrexate, do not have adverse effects on bone mass [18]. Anti-TNF therapy (infliximab) has shown positive effects on bone mass [19]. It is believed that the bone protective effect is not only due to the decreased disease activity, but also to the role played by the cytokine TNF in osteoclastogenesis [20,21]. Regarding tocilizumab (inhibitor of IL-6), its role is fundamental, since IL-6 intervenes in the inflammatory and osteoclastogenic process [13].
The CAMERA II study analyzed patients with early RA who were on methotrexate and 10 mg prednisone and were administered 500 mg of calcium, 400 IU of vitamin D and alendronate/risedronate over two years. BMD showed a 2.6% increase in lumbar spine bone mass during the first year of prophylactic treatment regardless of glucocorticoid use, demonstrating that the addition of 10 mg of prednisone daily in a methotrexate-based treatment does not lead to bone loss in patients with early RA [22].
The quality of life of these patients is obviously affected. A study by Riggs and Melton showed that a hip fracture has a mortality rate of 10-20% in the subsequent 6 months, 50% of these patients will not be able to walk without external objects such as walking sticks. Also 25% will need home help for long periods [23].
Systemic Lupus Erythematosus (SLE)
SLE is an autoimmune disease of unknown origin that occurs more frequently in young women [4]. Osteoporosis is also an associated disease, and probably caused by treatments that have a negative effect on the bone or by ovarian dysfunction induced by some immunosuppressants [24].
The loss of bone mass in SLE could be the result of several mechanisms, those that depend on the disease itself and those that are treatment related (Table 3).
The mechanism responsible for the loss of bone mass in patients with SLE receiving corticosteroids is the marked increase in bone resorption and the maturation deficit of the osteoblast, as well as bone mineralization. BMD begins to fall from the third month of glucocorticoid use and progresses rapidly to six months. From this period, the curve gradually declines [25].
A study by Houssiau et al in premenopausal women with a definitive diagnosis of SLE, showed that patients who had not received corticosteroids had lower BMD compared to healthy controls, indicating that the disease alone is a bone loss factor [26].
A systematic review by Wang et al showed that SLE patients have lower levels of BMD than the general population, and that not only occurs in a specific place, but there is loss of bone mass in all the places studied (femoral neck, lumbar spine and hip). In addition, an increased risk of fracture was demonstrated (RR=1.97) [27].
Jacobs et al, in a prospective 6-year follow-up study in patients with SLE, found an association between loss of BMD in the lumbar spine and the use of corticosteroids at high doses. In addition, a loss of bone mass was demonstrated with the use of immune-suppressants and base antimalarials [28].
Regarding the association between lower serum vitamin D levels in these patients and the loss of bone mass, the deficiency is believed to be due to reduced sun exposure by the photosensitivity of these patients, in addition to the use of corticosteroids and renal insufficiency [29]. The mechanism by which these patients have a lower calcium absorption results in a decrease in the conversion of 25(OH)-vitamin D to 1,25(OH)2-vitamin D (calcitriol). This association has been demonstrated in cross-sectional studies [29,30,31], hence the importance of determining levels of 25(OH)-vitamin D in patients with SLE.
Patients with SLE and neurological involvement (epilepsy, stroke, etc.) comprise the group at highest risk of fractures as they are more prone to falls and to the adverse effects of antiepileptics [32].
Ankylosing Spondylitis (AS)
AS is part of the group of seronegative spondylo-arthropathies (SpA), a group of inflammatory joint disorders that also includes psoriatic arthritis, inflammatory bowel disease and reactive arthritis. AS is the typical form of SpA with symptoms related to arthritis, enthesitis, sacroiliitis, among others. The location of the primary disease is believed to be enthesis, i.e. the area where the tendons and ligaments are inserted into the bone [4].
Axial involvement generates a negative bone impact and increases the risk of vertebral fracture due to continuous inflammation, which results in a decrease in bone mass and progressive ankylosis, together with bone proliferation [33].
Osteoporosis can occur due to physical inactivity, reduced mobility of the spine related to pain, stiffness and ankylosis, in addition to subclinical involvement of intestinal disease [34].
The prevalence of osteoporosis in AS is approximately 14-27% in the spine and 4-14% in the hip [35,36]. Using the visual and morphometric definitions of vertebral size, the prevalence of fractures in this group of patients is 10-30% [37,38].
Prieto et al demonstrated a strong association between AS and vertebral fractures. These patients have a 5-fold higher risk of fracture compared to a control group. In addition, they observed that the first 2.5 years of evolution of the disease are critical because the peak fracture risk increases and is believed to be secondary to the inflammatory [33]. These data are similar to those reported by Van der Wijden et al, suggesting that fractures in AS are associated with exacerbations of the disease. In addition, they showed that vertebral fractures were more frequent in men [39].
It is important to emphasize that this group of patients has a high risk of vertebral fractures. However, they do not present an increased risk of non-vertebral fractures.
Psoriatic Arthritis (PA)
PAs are also part of seronegative spondyloarthropathies. Studies on bone involvement in these patients are scarce, probably because the frequency of the disease is lower than that of rheumatoid arthritis and systemic lupus erythematosus. However, a relationship between inflammatory markers (TNF, IL-6, interferon-gamma or IFN-γ) and loss of bone mass have been demonstrated [40].
Keller et al showed that the group of patients with severe PAs have lower bone mass loss values than those with mild to moderate PA, showing that inflammatory factors play a fundamental role in bone resorption [40].
Dreiher et al analyzed 7,939 patients with psoriasis. The prevalence of osteoporosis was significantly higher compared to the control group and more frequent in women. However, it was observed that in the male group osteoporosis was the result of systemic disease (PAs), whereas in women it was shown to be due to an estrogen deficiency. In addition, women tended to be diagnosed before men because, usually, women are routinely referred for bone evaluation [41].
Osteoarthrosis (OA)
Osteoarthrosis is a degenerative disease that usually occurs over 45 years and whose origin is multifactorial, with joint disease being more frequent [4].
Arthrosis, together with osteoporosis, represent the two etiologies that affect the bone and joint structure in the elderly population and in turn are the primary cause of the deterioration of their quality of life [42].
Yoshimura et al carried out a 10-year follow-up study in patients with lumbar osteoarthritis and osteoporosis. The cumulative incidence of lumbar osteoarthritis in 10 years within the age range of 40-79 years was 25.8% in men and 45.2% in women. In turn, there was a significant relationship between the presence of lumbar osteoporosis and the incidence of lumbar osteoarthritis [42].
The inverse relationship of arthrosis and osteoporosis has been debated for years. Zhang et al demonstrated the mechanical and microstructural structures of the subchondral trabecular bone of postmenopausal women with arthrosis and osteoporosis, through the determination of fractional bone volume and bone mineral density. The group of patients with osteoarthritis showed a greater fractional bone volume compared to the group of patients with osteoporosis. However, the relationship with bone density was not significant [43].
Regarding the production of fractures in these patients, the causal factor is believed to be falls. A longitudinal global study of osteoporosis found that approximately 40% of the population studied had osteoarthritis, and the direct relationship with fractures was not significant after adjusting for incidental falls. However, the adjusted relative risk for osteoarthritis as a predictor of falls was 1.24 (95% CI, 1.22 - 1.26, p<0.0001). In addition, postmenopausal women with osteoarthritis were 25% more likely to fall than women without osteoarthritis [33].
Glucocorticoid-induced Osteoporosis (GIO)
GIO is the most common cause of secondary osteoporosis. Prolonged treatment with 2.5-5 mg daily of prednisolone has been shown to increase the risk of vertebral and hip fracture [16,44].
GIO most commonly affects the areas of trabecular bone (lumbar spine and proximal femur). Fractures can occur in approximately 30-50% of patients receiving corticosteroids on an extended basis [44].
Table 4 describes some risk factors for GIO.
Pathophysiology
Glucocorticoids have a direct and indirect effect on metabolism by blocking the actions of vitamin D and calcium absorption, leading to a decrease in serum calcium and an increase in parathormone (PTH) levels [32]. However, raised PTH does not fully account for bone loss from glucocorticoid exposure [45].
During the initial period of treatment with corticosteroids, bone resorption increases, resulting in a sudden decrease in bone density and increased risk of fracture [45].
The specific mechanism by which corticosteroids induce bone resorption is by activating the RANKL receptor and the macrophage colony stimulating factor (MCSF). These two components are a fundamental part of osteoclastogenesis, along with decreased OPG receptor [46].
As for bone formation, it is affected due to the inhibition of osteoblast precursor cells and an increase in osteoblastic apoptosis [45]. In addition, there is inhibition of the function of mature osteoblasts and suppression of growth factors (IGF1) in bone cells, together with increased apoptosis of osteocytes [47,48] (Table 5).
Evaluation
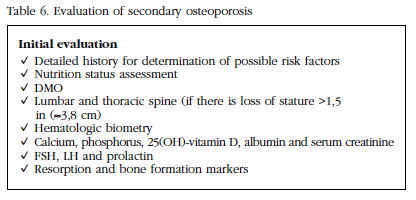
Patients with osteoporosis should be investigated for possible causes of bone loss (Table 6). During physical examination, it is important to measure the patient's height and compare with previous measurements, in order to evaluate possible asymptomatic vertebral fractures.
Treatment
It is common to find suboptimal levels of vitamin D and calcium in these patients. Because of this, a correction treatment of these levels should be implemented.
In patients who begin treatment with glucocorticoids for more than 3 months at doses >5 mg/day, it is necessary to establish preventive treatment for bone mass loss, and in those patients who are already being treated, a bone densitometry should be carried out to assess the reduction of bone mass and the possible risk of fracture [17,49].
Due to increased use of corticosteroids and the prevalence of secondary osteoporosis, several international societies have described treatment guidelines. However, there is no global consensus on this matter [50,51,52,53]. In 2010 the ACR (American College of Rheumatology) published guidelines for the management of GIO, according to FRAX® (Figures 2 and 3) [54].

Figure 2 Management of patients of both genders> 50 years who are initiating or receiving glucocorticoid (GC) therapy
Non-pharmacological treatment
The management and prevention of GIO in non-pharmacological treatment is similar to that of primary osteoporosis and consists in eliminating modifiable risk factors [55]:
- Smoking.
- Alcohol consumption (≥3 unit/day).
- Sedentarism (energy consumption ≤1,682 kcal/day).
- Diet high in sodium (mainly in the presence of hypercalciuria).
- Decreased body mass index.
Patients with advanced age require special help to avoid falls, which are the main risk factor for fractures.
Calcium
Calcium and vitamin D are nutritional elements considered essential in any therapeutic option for osteoporosis.
The use of calcium carbonate at a dose of 1,000 mg/day has not been shown to prevent bone loss or reduce the risk of fracture in patients initiating prolonged corticosteroid therapy, particularly in postmenopausal women. Because of this, it is not indicated for primary prevention [56]; however, secondary prevention has shown that BMD levels remain constant in the lumbar spine in postmenopausal women with the use of 500 mg/day of calcium carbonate accompanied by 0.25 µg/day of calcitriol [55].
Vitamin D
Active forms of vitamin D (alphacalcidol and calcitriol) and non-active forms (cholecalciferol and ergocalciferol) prevent the loss of bone mass in chronic glucocorticoid users [55].
Calciferol use prevents the reduction of bone mass, but does not reduce the incidence of fractures [57]. In contrast to non-active forms, the use of alfacalcidol at a dose of 0.25-1.0 µm/day plus 500 mg/day of calcium prevents the reduction of bone mass and reduces the risk of fracture (vertebral and non-vertebral) [58].
The combination of calcium and alphacalcidol has been the only one that has demonstrated a significant reduction in the risk of vertebral fracture. However, it shows no effect on non-vertebral fractures [58].
Randomized studies have shown that a dose of 700-800 IU of vitamin D reduces the risk of hip fracture and non-vertebral fractures in elderly patients [59]. For adults over 50, the National Osteoporosis Foundation recommends 800-1,000 IU of vitamin D per day. However, some experts recommend 1,000-2,000 IU daily, with a safety limit of 4,000 IU/day [60].
Bisphosphonates
The use of bisphosphonates has shown a positive effect on the loss of bone mass in patients who have been treated with glucocorticoids for prolonged periods [55].
In 3-to-5-year observational controlled studies, bisphosphonates have been shown to reduce vertebral and non-vertebral fractures including hip fracture [61]. In a meta-analysis carried out by Kanis et al, a significant reduction of non-vertebral fractures was demonstrated in comparison with the control group [62].
The use of bisphosphonates is recommended during the first two years of GIO, but there is insufficient evidence for long-term treatment [61].
In recent years, bisphosphonates have been the drugs of choice most commonly used to treat osteoporosis. Approved bisphosphonates for the treatment of glucocorticoid-induced osteoporosis are: etidronate, alendronate, risedronate and zoledronic acid. Contraindications to therapy include hypersensitivity or hypocalcemia; (≤30 ml/min of glomerular filtration rate for risedronate or ibandronate and ≤35 ml/min for alendronate and zoledronate) should be managed under surveillance [63].
The use of alendronate 5-10 mg/day for 48 weeks has been shown to increase bone mass [64,65]. A study by Adachi et al demonstrated an increase in lumbar spine bone mineral density by 2.8% (5 mg/day) and 3.9% (10 mg/day) in patients with prolonged glucocorticoid therapy [66]. A dose of 5 mg/day of risedronate increases bone mass and also reduces the risk of fracture [67].
Zoledronic acid is approved by the Food and Drug Administration for the treating and preventing osteoporosis in men and postmenopausal women as well as glucocorticoid-induced osteoporosis. The appropriate dose of zoledronic acid is 5 mg intravenously infused once a year, which has been shown to reduce the risk of spine fracture, non-vertebral fracture and hip fracture in postmenopausal women with osteoporosis [68].
Teriparatide
Teriparatide is an analog of PTH obtained by the recombinant DNA technique (PTH-1-34). This analogous agent increases osteoblastic function and decreases the apoptosis of osteocytes [55].
The use of teriparatide at a dose of 20 mg/day subcutaneously should be considered as a treatment for GIO because it significantly increases bone mineral density in this group of patients, in addition to reducing vertebral fractures. However, it has no effect on non-vertebral fractures [69].
Because of the high cost of teriparatide, its use is recommended when bisphosphonates fail [69], that is, when despite treatment with bisphosphonates, the bone mass continues to decrease, against fractures in the presence of bisphosphonates, or if they are contraindicated [55].
Denosumab
Denosumab is an antibody against RANKL, which is used to treat primary osteoporosis. However, the study by Dore et al in patients with rheumatoid arthritis under treatment with GC, demonstrated an increase in BMD and reduced resorption, compared to placebo [70].
As denosumab is not filtered by the kidneys, it may be a therapeutic option for patients with renal dysfunction who do not tolerate bisphosphonates [70].
The use of denosumab for the treatment of osteoporosis induced by GC has not been approved yet, and its study is in stage III [71]. More evidence is needed to prove its utility in GIO [61].
Odanacatib
Odanacatib is a protease cathepsin-K inhibitor which induces bone deterioration by the osteoclast [72]. A phase II study by Bone et al, using odanacatib 50 mg once weekly, demonstrated an increase in bone mineral density in the lumbar spine (5.5% vs. 0.2% in the control group) and hip 5.5% (3.2% vs 0.9% in the control group) [73]. However, there are not enough controlled studies to determine the proper use of this drug in GIO, in addition to not being approved by the FDA so far.
Sclerostin Inhibitors
The mechanism responsible for the inhibition of bone formation in patients with prolonged glucocorticoid therapy is the reduction of the half-life and activity of osteoclasts. Glucocorticoids have been shown to alter bone cell formation by reducing the proliferation of osteoblasts and suppressing growth factors [49]. Because of this, new therapeutic approaches are based on maintaining the viability of osteoblasts and osteocytes in the presence of glucocorticoids.
Sclerostin (Sost) is a protein produced by osteocytes and its main function is to inhibit the maturation of osteoblasts [74,75,76]. A study by Yao et al demonstrated an increase in sclerostin gene expression in patients exposed to GC therapy for 28 days [77].
Monoclonal antibodies to sclerostin (Scl-Ab) currently known as romosozumab (AMG 785), blosozumab and BPS804 have been developed which inhibit the activity of sclerostin and thus stimulate bone formation [76,78].
Yao et al used Scl-Ab in mice exposed to 4 mg/kg/day of methylpredonoxone, and showed bone volumes of trabecular bone (Tb-BV/TV) in lower lumbar spine and neck of femur, less mass cortical bone in the middle third of the femur and lower cortical bone resistance compared to the placebo group. In addition, models receiving 25 mg/kg of Scl-Ab had a 60-125% increase in Tb-BV/TV and an increase in vertebral resistance of 30-70% [79].
Studies to date have shown that sclerostin inhibitors may be a future therapeutic option for managing patients with severe osteoporosis [80].
Conclusion
Osteoporosis is a systemic skeletal disease characterized by decreased bone mineral density with alterations in bone microarchitecture and increased risk of fracture.
Glucocorticoids are the primary cause of secondary osteoporosis, being an independent factor of morbidity and mortality in these patients, because the progressive loss of bone mass and increased risk of fracture begins shortly after the start of treatment with glucocorticoids.
It is important to identify, and, if possible, to correct risk factors and comorbidities in this group of patients, initiate preventive measures and provide health promotion tips such as change of habits, and give calcium and vitamin D supplements, in addition to treatment specific.
Referencias
1 Khosla S, Luftkin E, Hodgson S, Fitzpatrick L, Melton LJ 3rd. Epidemiology and clinical features of osteoporosis in young individuals. Bone. 1994;15(5):551-5. [ Links ]
2 Walsh L, Wong C, Pringle M, Tattersfield A. Use of oral corticosteroids in the community and the prevention of secondary osteoporosis: a cross sectional study. BMJ. 1996;313:344-6. [ Links ]
3 Sinigaglia L. Epidemiology of osteoporosis in rheumatic diseases. Rheum Dis Clin North Am. 2006;631-58. [ Links ]
4 Firestein G, Budd R, Gabriel S, McIness I, O’Dell J. Kelley’s Textbook of Rheumatology. 9th ed. Elsevier; 2013. 2292 p. [ Links ]
5 Baker-LePain J, Nakumura M, Lane N. Effects of Inflammation on bone: an update. Curr Opin Rheumatol. 2011;23(4):389-95. [ Links ]
6 Walsh N, Crotti T, Goldring S, Gravallese EM. Rheumatic diseases: the effect of inflammation on bone. Immunol Rev. 2005;208:228-51. [ Links ]
7 Rifas L, Weitzmann M. A novel T cell cytokine, secreted osteoclastogenic factor of activated T cells, induces osteoclast formation in a RANKL-independent manner. Arthritis Rheum. 2009;60:3324-35. [ Links ]
8 Kong Y, Feige U, Sarosi I, Bolon B, Tafuri A, Morony S, et al. Activated T cells regulate bone loss and joint destruction in adjuvant arthritis through osteoprotegerin ligand. Nature. 1999;402:304-9. [ Links ]
9 Jódar Gimeno E. Conclusiones consensuadas del I Foro Multidisciplinar en el Manejo del Paciente con Alto Riesgo de Fractura (ARF) Osteoporótica. Rev Osteoporos Metab Miner. 2010;2(2):79-86. [ Links ]
10 Moon SJ, Ahn IE, Kwok SK, Park KS, Min JK, Park SH, et al. Periarticular osteoporosis is a prominent feature in early rheumatoid arthritis: estimation using shaft to periarticular bone mineral density ratio. Korean Med Sci. 2013;28:287-94. [ Links ]
11 Forsblad D, Larsen A, Waltbrand E, Kvist G, Mellstrom D, Saxne T, et al. Radiographic joint destruction in postmenopausal rheumatoid arthritis is strongly associated with generalised osteoporosis. Ann Rheum Dis. 2003;62:617-623. [ Links ]
12 Van Staa T, Geusens P, Bijlsma J, Leufkens H, Cooper C. Clinical assessment of the long-term risk of fracture in patients with rheumatoid arthritis. Arthritis Rheum. 2006;54(10):3104-12. [ Links ]
13 Edwards CJ, Williams E. The role of interleukin-6 in rheumatoid arthritis-associated osteoporosis. Osteoporos Int. 2010;21(8):1287-93. [ Links ]
14 Sinigaglia L, Nevertti A, Mela Q. A multicenter cross-sectional study on bone mineral density in rheumatoid arthritis. J Rheumatol. 2000;27:2528-9. [ Links ]
15 Stafford L, Bleasel J, Giles A. Androgen deficiency and bone mineral density in men with rheumatoid arthritis. J Rheumatol. 2000;27:2786-90. [ Links ]
16 Van Staa T, Leufkens H, Cooper C. The epidemiology of corticosteroid-induced osteoporosis: a meta-analysis. Osteoporos Int. 2002;13:777-87. [ Links ]
17 Deal C. Recent recommendations on steroid-induced osteoporosis: more targeted but more complicated. Clevel Clin J Med. 2013;80(2):117-25. [ Links ]
18 Di Munno O, Mazzantini M, Sinigaglia L, Bianchi G, Minisola G, Muratore M, et al. Effect of low-dose methotrexate on bone density in women with rheumatoid arthritis: results from a multicenter cross-sectional study. J Rheumatol. 2004;31:1305-9. [ Links ]
19 Seriolo B, Paolino S, Sulli A, Ferretti V, Cutolo M. Bone metabolism changes during anti-TNF-alpha therapy in patients with active rheumatoid arthritis. Ann NY Acad Sci. 2006;1069:420-7. [ Links ]
20 Marotte H, Pallot-Prades B, Grange L, Gaudin P, Alexandre C, Miossec P. A 1-year case–control study in patients with rheumatoid arthritis indicates prevention of loss of bone mineral density in both responders and nonresponders to infliximab. Arthritis Res Ther. 2007;9(3):R61. [ Links ]
21 Lange U, Teichmann J, Müller-Ladner U, Strunk J. Increase in bone mineral density of patients with rheumatoid arthritis treated with anti-TNF-alpha antibody: a prospective open-label pilot study. Rheumatol. 2005;44:1546-8. [ Links ]
22 Van der Goes M, Jacobs G, Jurgens M, Bakker MF, van der Veen MJ, van der Werf JH, et al. Are changes in bone mineral density different between groupsof early rheumatoid arthritis patients treated according to a tight control strategy with or without prednisone if osteoporosis prophylaxis is applied? Osteoporos Int. 2013;24:1429-36. [ Links ]
23 Riggs B, Melton LJ 3rd. The worldwide problem of osteoporosis: insights afforded by epidemiology. Bone. 1995;115:505S-11S. [ Links ]
24 Mok C, Mak A, Ma K. Bone mineral density in post menopausal Chinese patients with Systemic Lupus Erythematosus. Lupus. 2005;14:106-12. [ Links ]
25 Lai CC, Chen WS, Chang DM, Tsao YP, Wu TH, Chou CT, et al. Increased serum fibroblast growth factor-23 and decreased bone turnover in patients with systemic lupus erythematosus under treatment with cyclosporine and steroid but not steroid only. Osteoporos Int. 2015;26(2):601-10. [ Links ]
26 Houssiau F, Lefebvre C, Depresseux G, Lambert M, Devogelaer JP, Nagant de Deuxchaisnes C. Trabecular and cortical bone loss in systemic lupus erythematosus. Br J Rheumatol. 1996;35:244-7. [ Links ]
27 Wang X, Yan S, Liu C, Xu Y, Wan L, Wang Y, et al. Fracture risk and bone mineral density levels in patients with systemic lupus erythematosus: a systematic review and meta-analysis. Osteoporos Int. 2016;4:1413-23. [ Links ]
28 Jacobs J, Korswagen LA, Schilder AM, Van Tuyl LH, Dijkmans BAC, Lems WF, et al. Six-year follow-up study of bone mineral density in patients with systemic lupus erythematosus. Osteoporos Int. 2013;24(6):1827-33. [ Links ]
29 Bultink E, Lems W, Kostense P, Dijkmans BA, Voskuyl AE. Prevalence and risk factors for low bone mineral density and vertebral fractures in patients with systemic lupus erythematosus. Arthritis Rheum. 2005;54:2044-50. [ Links ]
30 Toloza S, Cole D, Gladman D, Ibanez D, Urowitz M. Vitamin D insufficiency in a large female SLE cohort. Lupus. 2010;19:13-9. [ Links ]
31 Ruiz-Irastorza G, Egurbide M, Olivares N, Martinez-Berriotxoa A, Aguirre C. Vitamin D deficiency in systemic lupus erythematosus: prevalence, predictors and clinical consequences. Rheumatology. 2008;47:920-3. [ Links ]
32 Mazziotti G, Canalis E, Giustina A. Drug-induced osteoporosis: mechanisms and clinical implications. Am J Med. 2010;123:877-84. [ Links ]
33 Prieto-Alhambra D, Nogues X, Javaid K, Wyman A, Arden NK, Azagra R, et al. An increased rate of falling leads to a rise in fracture risk in postmenopausal women with self-reported osteoarthritis: a prospective multinational cohort study (GLOW). Ann Rheum Dis. 2013;72:911-7. [ Links ]
34 Ralston S, Urquhart G, Brezeski M, Sturrock RD. Prevalence of vertebral compression fractures due to osteoporosis in ankylosing spondylitis. BMJ. 1990;300:563-5. [ Links ]
35 Lange U, Kluge A, Strunk J, Teichmann J, Bachmann G. Ankylosing spondylitis and bone mineral density. What is the ideal tool for measurement? Rheumatol Int. 2005;26(2):115-20. [ Links ]
36 Cooper C, Carbone L, Michet C, Atkinson EJ, O'Fallon WM, Melton LJ 3rd. Fracture risk in patients with ankylosing spondylitis:a population based study. J Rheumatol. 1994;21:1877-82. [ Links ]
37 Mullaji A, Upadhyay S, Ho E. Bone mineral density in ankylosing spondylitis. DEXA comparison of control subjects with mild and advanced cases. J Bone Jt Sur Br. 1994;76:660-5. [ Links ]
38 Toussirot R, Michel F, Wendling D. Bone density, ultrasound measurements and body composition in early ankylosing spondylitis. Rheumatol. 2001;40:882-8. [ Links ]
39 van der Weijden M, van der Horst-Bruinsma I, van Denderen J, Dijkmans B, Heymans M, Lems W. High frequency of vertebral fractures in early spondylarthropathies. Osteoporos Int. 2012;23:1683-90. [ Links ]
40 Keller J, Kang J, Lin H. Association between osteoporosis and psoriasis: results from the Longitudinal Health Insurance Database in Taiwan. Osteoporos Int. 2013;24:1835-41. [ Links ]
41 Dreiher J, Weirzman D, Cohen A. Psoriasis and osteoporosis: a sex-specific association? J Inves Dermatol. 2009;129:1643-9. [ Links ]
42 Yoshimura N, Muraki S, Oka H, Mabuchi A, Kinoshita H, Yosihda M, et al. Epidemiology of lumbar osteoporosis and osteoarthritis and their causal relationship-is osteoarthritis a predictor for osteoporosis or vice versa?: the Miyama study. Osteoporos Int. 2009;20(6):999-1008. [ Links ]
43 Zhang ZM, Li ZC, Jiang LS, Jiang SD, Dai LY. Micro-CT and mechanical evaluation of subchondral trabecular bone structure between postmenopausal women with osteoarthritis and osteoporosis. Osteoporos Int. 2010;21(8):1383-90. [ Links ]
44 Pereira RM, Carvalho JF, Paula AP, Zerbini C, Domiciano DS, Gonçalves H, et al. Guidelines for the prevention and treatment of glucocorticoid-induced osteoporosis. Rev Bras Reum. 2012;52(4):569-93. [ Links ]
45 Mirza F, Canalis E. Secondary osteoporosis: Pathophysiology and management. Eur J Endocrinol. 2015;173(3):R131-51. [ Links ]
46 Hofbauer L, Gori F, Riggs B, Lacey D, Dunstan C, Spelsberg T, et al. Stimulation of osteoprotegerin ligand and inhibition of osteoprotegerin production by glucocorticoids in human osteoblastic lineage cells: potential paracrine mechanisms of glucocorticoid- induced osteoporosis. Endocrinology. 1999;140:4382-9. [ Links ]
47 Dallas S, Prideaux M, Bonewald L. The osteocyte: an endocrine cell... and more. Endocr Rev. 2013;34:658-90. [ Links ]
48 Delany A, Durant D, Canalis E. Glucocorticoid suppression of IGF I transcription in osteoblasts. Mol Endocrinol. 2001;15(10):1781-9. [ Links ]
49 Weinstein R. Glucocorticoid-Induced Bone Disease. New Eng J Med. 2011;365:62-70. [ Links ]
50 American College of Rheumatology. Recommendations for the prevention and treatment of glucocorticoid-induced osteoporosis: 2001 update. American College of Rheumatology Ad Hoc Committee on Glucocorticoid-Induced Osteoporosis. Arthritis Rheum. 2001;44:1496-503. [ Links ]
51 Adler R, Hochberg M. Suggested guidelines for evaluation and treatment of glucocorticoid-induced osteoporosis for the Department of Veterans Affairs. Arch Intern Med. 2003;163(21):2619-24. [ Links ]
52 Devogelaer J, Goemaere S, Boonen S, Body J, Kaufman J, Reginster JY, et al. Evidence-based guidelines for the prevention and treatment of glucocorticoid-induced osteoporosis: a consensus document of the Belgian Bone Club. Osteoporos Int. 2006;17(1):8-19. [ Links ]
53 Messina O, Somma L, Tamborenea M, Castelli G, Riopedre A, Lancioni G, et al. Guías para el diagnóstico, la prevención y el tratamiento de la osteoporosis inducida por glucocorticoides en el adulto. Actual Osteol. 2016;12(2):107-25. [ Links ]
54 Grossman J, Gordon R, Ranganath V, Deal C, Caplan L, Chen W, et al. American College of Rheumatology 2010 recommendations for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Care Res. 2010;62(11):1515-26. [ Links ]
55 Rodrigues Pereira R, Freire de Carvalho J, Paula A, Zerbini C, Domiciano D, Gonçalves H, et al. Guidelines for the prevention and treatment of glucocorticoid-induced osteoporosis. Rev Bras Reum. 2012;52(4):569-93. [ Links ]
56 Sambrook P, Birmingham J, Kelly P, Kempler S, Nguyen T, Pocock N, et al. Prevention of corticosteroid osteoporosis. A comparison of calcium, calcitriol and calcitonin. N Eng J Med. 1993;328(24):1747-52. [ Links ]
57 Richy F, Ethgen O, Bruyere O, Regunster J. Efficacy of alfacalcidol and calcitriol in primary and corticosteroid-induced osteoporosis: a meta-analysis of their effects on bone mineral density and fracture rate. Osteoporos Int. 2004;14(5):301-10. [ Links ]
58 Ringe JD, Dorst A, Faber H, Schacht E, Rahlfs V. Superiority of alfacalcidol over plain vitamin D in the treatment of glucocorticoid- induced osteoporosis. Rheumatol Int. 2004;24(2):63-70. [ Links ]
59 Bischoff-Ferrari H, Willett W, Wong JB, Giovannucci E, Dietrich T, Dawson-Hughes B. Fracture prevention with vitamin D supplementation: a meta-analysis of randomized controlled trials. JAMA. 2005;293:2257-64. [ Links ]
60 Ross A, Manson J, Abrams S, Aloia JF, Brannon PM, Clinton SK, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96(1):53-8 [ Links ]
61 Lems W, Saag K. Bisphosphonates and glucocorticoid-induced osteoporosis: cons. Endocrine. 2015;49(3):628-34. [ Links ]
62 Kanis JA, Stevenson M, McCloskey EV, Davis S, Lloyd-Jones M. Glucocorticoid-induced osteoporosis: a systematic review and cost-utility analysis. Health Technol Assess. 2007;11(7):1-231. [ Links ]
63 Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorders (CKD-MBD). Kidney Int Suppl. 2009;76(113):S1-130. [ Links ]
64 Saag K, Emkey R, Schnitzer T, Brown J, Hawkins F, Goemacre S, et al. Alendronate for the prevention and treatment of glucocorticoid- induced osteoporosis. N Eng J Med. 1998;339(5):292-9. [ Links ]
65 de Nijs R, Jacobs J, Lems W, Laan R, Algra A, Huisman A, et al. Alendronate or alfacalcidol in glucocorticoid-induced osteoporosis. N Engl J Med. 2006;355(7):675-84. [ Links ]
66. Adachi JD, Saag KG, Delmas PD, Liberman UA, Emkey RD, Seeman E, et al. Two-year effects of alendronate on bone mineral density and vertebral fracture in patients receiving glucocorticoids: a randomized, double-blind, placebo-controlled extension trial. Arthritis Rheum. 2001;44(1):202-11. [ Links ]
67 Cohen S, Levy R, Keller M, Boling E, Emkey R, Greenwald M, et al. Risedronate therapy prevents corticosteroid-induced bone loss: a twelve-month, multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Arthritis Rheum. 1999;42(11):2309-18. [ Links ]
68 Watts N, Bilezikian J, Camacho P, Greenspan S, Harris S, Hodgson S, et al. American Association Of Clinical Endocrinologists Medical Guidelines for clinical practice for the diagnosis and treatment of postmenopausal osteoporosis: executive summary of recommendations. Endocr Pr. 2010;16(6):1016-9. [ Links ]
69 Saag K, Shane E, Boonen S, Marín F, Donley D, Taylor K, et al. Teriparatide or alendronate in glucocorticoid-induced osteoporosis. N Engl J Med. 2007;357(20):2028-39. [ Links ]
70 Dore RK, Cohen SB, Lane NE, Palmer W, Shergy W, Zhou L, et al. Effects of denosumab on bone mineral density and bone turnover in patients with rheumatoid arthritis receiving concurrent glucocorticoids or bisphosphonates. Ann Rheum Dis. 2010;69(5):872-5. [ Links ]
71 Whittier X, Saag K. Glucocorticoid-induced Osteoporosis. Rheum Dis Clin N Am. 2016;42(1):177-89. [ Links ]
72 Boonen S, Rosenberg F, Claessens F, Vanderschueren D, Papapoulos S. Inhibition of cathepsin K for treatment of osteoporosis. Curr Osteoporos Rep. 2012;10(1):73-9. [ Links ]
73 Bone H, McClung MR, Roux C, Recker RR, Eisman JA, Verbruggen N, et al. Odanacatib, a cathepsin K inhibitor for osteoporosis: a two-year study in post- menopausal women with low bone density. J Bone Miner Res. 2010;25:937-47. [ Links ]
74 Paszty C, Turner C, Robinson M. Sclerostin: a gem from the genome leads to bone-building antibodies. J Bone Min Res. 2010;25(9):1897-904. [ Links ]
75 van Bezooijen R, Roelen B, Visser A, van der Wee-Pals L, de Wilt E, Karperien M, et al. Sclerostin is an osteocyte-expressed negative regulator of bone formation, but not a classical BMP antagonist. J Exp Med. 2004;199(6):805-14. [ Links ]
76 Li X, Warmington K, Niu Q, Asuncion F, Barrero M, Grisanti M, et al. Inhibition of sclerostin by monoclonal antibody increases bone formation, bone mass and bone strength in aged male rats. J Bone Min Res. 2010;25(12):2647-56. [ Links ]
77 Yao W, Cheng Z, Busse C, Pham A, Nakamura M, Lane N. Glucocorticoid excess in mice results in early activation of osteoclastogenesis and adipogenesis and prolonged suppression of osteogenesis: a longitudinal study of gene expression in bone tissue from glucocorticoid-treated mice. Arthritis Rheum. 2008;58(6):1674-86. [ Links ]
78 McClung M, Grauer A, Boonen S, Bolognese M, Brown J, Diez-Perez A, et al. Romosozumab in postmenopausal women with low bone mineral density. New Eng J Med. 2014;370(5):412-20. [ Links ]
79 Yao W, Dai W, Jiang L, Lay EY, Zhong Z, Ritchie R, et al. Sclerostin-antibody treatment of glucocorticoid-induced osteoporosis maintained bone mass and strength. Osteoporos Int. 2016;27:283-94. [ Links ]
80 Appelman-Dijkstra N, Papapoulos S. Sclerostin inhibition in the management of osteoporosis. Calcif Tissue Int. 2016;98:370-80. [ Links ]











 text in
text in