My SciELO
Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista de Osteoporosis y Metabolismo Mineral
On-line version ISSN 2173-2345Print version ISSN 1889-836X
Rev Osteoporos Metab Miner vol.10 n.2 Madrid Apr./Jun. 2018 Epub May 17, 2021
https://dx.doi.org/10.4321/s1889-836x2018000200005
ORIGINALS
Monthly versus biweekley calcifediol in the treatment of osteoporotic patients. Study in real life
1Departamento de Medicina Interna. Hospital Universitario Marqués de Valdecilla-Instituto de Investigación Sanitaria (IDIVAL) - Santander (España)
1Universidad de Cantabria - Santander (España)
2Servicio de Aparato Digestivo. Hospital Universitario Marqués de Valdecilla-Instituto de Investigación Sanitaria (IDIVAL) - Santander (España)
Objectives:
To assess serum concentrations of 25-hydroxyvitamin D, 25(OH)D, in osteoporotic patients treated for one year with calcifediol.
Methods:
We have studied 156 patients with osteoporosis (23 males and 133 females), aged 71,9±9,6 years who had received treatment with calcifediol for at least one year. Ninety-two of them received 0.266 mg of calcifediol every fifteen days and the remaining 64 the same dose once a month. Serum levels of 25(OH)D, intact PTH (iPTH), procollagen type 1 amino-terminal propeptide (PINP) and C-terminal crosslinked telopeptide of type I collagen (CTX) were determined before and one year after starting treatment.
Results:
A significant increase in the concentration of 25(OH)D was observed with both treatment regimens (p<0.001). The percentage of patients who reached levels of 25(OH)D higher than 20 and 30 ng/ml was similar with both guidelines, while the percentage of patients exceeding 60 ng/ml was higher with the biweekly dose (p<0.01). The concentration of iPTH decreased significantly after the administration of calcifediol, although on this occasion there were no differences between the two forms of treatment. Both bone remodeling markers, PINP and CTX, decreased similarly in patients treated with antiresorptives (p<0.0001), without these changes being related to the calcifediol regimen.
Introduction
Opinions vary as to what constitutes normal levels of vitamin D in the general population1-5. According to some authors, severe vitamin D deficiency is when serum levels of 25-hydroxyvitamin D (25(OH)D or calcifediol) are below 10 ng/mL, moderate deficiency when they are between 10 and 20 ng/mL, insufficiency between 20 and 30 ng/mL and adequate values if they are above 30 ng/mL1-3. Others, such as the US Institute of Medicine, propose values for the healthy general population above 20 ng/mL4. Nor is there an established excessive, potentially harmful level, although there is a tendency to set this at around 50-60 ng/mL1,5,7,8.
As for patients with osteoporosis, most clinical practice guidelines indicate that treatment with antiresorptive or anabolic drugs must be accompanied by an adequate supply of vitamin D, in addition to an appropriate amount of calcium9-11. The latter, whenever possible, should be administered through diet, while vitamin D is recommended to be administered as supplements. An aspect also debated is the serum 25(OH)D levels that patients with this disease should reach, although most authors and scientific societies, including Spanish ones, recommend serum concentrations above 30 ng/mL2,3,5,6,9-12. To achieve these aims, a daily dose of 800-1,000 IU of vitamin D (in Europe the vitamin D used is vitamin D3 –cholecalciferol–) is advised, although its weekly, biweekly or monthly equivalent can also be administered10. The biological potency of vitamin D is established as international units (IU), so that 1 µg of cholecalciferol equals 40 IU13. In some countries, as in the case of Spain, 25-hydroxyvitamin D3, a metabolite of vitamin D, also called calcifediol or calcidiol, is also commercially available. It is prepared in dispensing units containing 0.266 mg. The exact equivalence of this dose with vitamin D in terms of metabolic activity (how many IU of vitamin D activity is 1 µg of calcifediol) is not known with total precision, but the most widespread therapeutic regimen has been to administer once to the month or every fifteen days, compared to the 800-1,000 IU of cholecalciferol daily, as mentioned above.
Calcifediol is more hydrophilic than cholecalciferol, with a shorter half-life, and causes a more rapid, sustained increase in serum levels of 25(OH)D14,15. A recent study carried out in our country in 40 women with postmenopausal osteoporosis16 reported 25(OH)D levels around 80 ng/mL (basal: 15.2 ng/mL) after the administration of a weekly dose of 0.266 mg of calcifediol for six months, and around 65 ng/mL (basal: 15.8 ng/mL) with the same dose in a fortnightly schedule. In another study carried out by our group17 in osteoporotic women receiving treatment with alendronate, the administration of 0.266 mg of calcifediol weekly for three months also managed to increase the levels of 25(OH)D in a similar way (82±31 ng/mL basal 21 ng/mL).
These data suggest that the 25(OH)D levels reached with the weekly or biweekly therapeutic regimen could generate concentrations higher than the desirable levels of vitamin D1,5-8. It is therefore possible to think that administering a more spaced dose of calcifediol over time is sufficient to maintain adequate levels of 25(OH)D in routine clinical practice. Thus, we have proposed: a) to evaluate the serum concentrations of 25(OH)D in osteoporotic patients treated for a year with biweekly or monthly doses of 0.266 mg of calcifediol; and b) determine if there are changes in intact parathyroid hormone levels (iPTH) and remodeling markers, amino-terminal propeptide of type I procollagen (PINP) and carboxyterminal telopeptide of type I collagen (CTX), after administration of both doses of calcifediol.
Material and methods
Patients
We retrospectively reviewed the data of the last 200 osteoporotic patients who had been treated until the time of study in the Bone and Mineral Metabolism Unit of our Center and who had received treatment with 0.266 mg of calcifediol (Hydroferol®) every two weeks or monthly for at least one year (mean ± SD: 15±3 months). Exclusion criteria were the suffering of certain processes (malabsorption, renal failure [Glomerular filtration <45 mL/min/1.73 m2], uncontrolled hyperthyroidism, primary hyperparathyroidism, chronic liver diseases, chronic inflammatory diseases) or the follow-up of treatments that they could interfere with bone metabolism (glucocorticoids, anticonvulsants, antihormonal treatments), as well as previous treatment with vitamin D supplements. Those who recognized inadequate therapeutic compliance were also excluded. The diagnosis of osteoporosis was based on the presence of fragility fractures (vertebral or hip) or the existence of a bone densitometry with T≤2.5 values in the lumbar spine, femoral neck or total hip (DXA, Hologic QDR 4500). The majority of patients also received treatment with antiresorptives (63% with oral bisphosphonates, 9% with zoledronate and 28% with denosumab). Clinical and analytical data were collected through the electronic medical record. In all cases, the month in which the two analytical determinations were made (baseline and after treatment) was collected. The body mass index (BMI) was obtained by dividing the weight in kg. between the height in meters squared. Weight and height were measured while the patient was in underwear and without shoes.
The study was approved by our clinical research ethics committee (CEIC) (2017.023).
The criterion for deciding the therapeutic regimen, biweekly or monthly, was based on the baseline of 25(OH)D, preferring the first in cases in which this figure was lower. However, a precise cutting value was not established, leaving the decision to the opinion of the doctor responsible for the patient. The retrospective analysis showed that 90% of the patients who received the biweekly regimen had baseline values lower than 20 ng/mL, while this percentage was only 53% in the case of the monthly regimen.
Analytical determinations
Serum levels of 25(OH)D, iPTH, PINP and CTX were determined before (baseline) and at least one year (12-25 months) after starting treatment with calcifediol (mean ± SD: 15±3 months).
All determinations were made on an empty stomach early in the morning. The routine determinations (glucose, creatinine, calcium, albumin, phosphate, alkaline phosphatase) were carried out by automated methods in a TechniconDax autoanalyzer (Technicon Instruments, Colorado, USA). The serum concentrations of 25(OH)D, iPTH and markers of remodeling (PINP and CTX) were carried out by an automated chemiluminescence system. In the case of 25(OH)D, the chemiluminescence assay of DiaSorin LIAISON (DiaSorin, Stillwater, Minnesota, USA) was used, and in the remaining tests the IDS-ISYS assay (Immunodiagnostic Systems Hokding PLC, London, RU). The limit of detection of 25(OH)D was 4 ng/mL and the intraand inter-assay variation coefficients (CV) of 4.4% and 8.3%, respectively. Regarding iPTH, the limit of detection was 6 pg/mL, with a normal range of 10 to 45 pg/mL. The intra-assay and inter-assay CVs were 3.7% and 5.4%, respectively. The detection limit of the PINP was 5 ng/mL (reference range between 18 and 102 ng/mL) and its intra-assay and inter-assay CV of 4.6% and 9.2%, respectively. Finally, CTX intra-assay and inter-assay CVs were 5.9% and 10% respectively, and their reference range was 0.152-0.761 ng/mL.
Statistical study
The quantitative variables were expressed as mean ± SD or median (interquartile range) and were compared using Student's t test or Mann-Whitney U test, according to the distribution of the data. The qualitative variables were expressed as number and percentage, and for comparison the χ2 test or Fisher's test was used, as appropriate. The differences between the basal and annual values of calcitrope hormones and markers of remodeling were analyzed using the Wilcoxon test. The association between the percentage of change (baseline-annual) of serum levels of 25(OH)D, iPTH, PINP and CTX, and age, sex, BMI, weight and month of the year of the determination of the laboratory was analyzed using the Pearson/Spearman correlation coefficient. Data analysis was carried out using the SPSS v20 statistical package (Chicago, IL). A p<0.05 was considered significant in all calculations.
Results
After applying the exclusion criteria, we finally studied 156 patients (23 men and 133 women) between the ages of 49 and 93 years (mean ± SD: 71.9±9.6). All had received treatment with calcifediol for at least one year, with an average of 15±3 months. Ninety-two of them had received 0.266 mg of calcifediol every fortnight and the remaining 64 received the same dose once a month (Table 1). The patients who received the biweekly regimen were older (4.6 years older, p<0.05). There were, however, no differences in weight or BMI.
Table 1. Baseline characteristics of the patients included in the study (biweekly dose and monthly dose)
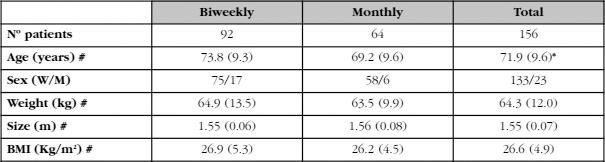
#: mean (SD); *: p<0,05.
As can be seen in table 2, and in accordance with the criterion that had led to the choice of one or another therapeutic regimen, the baseline levels of 25(OH)D were lower in patients who received fortnightly calcifediol than in those who received the monthly dose (p<0.01). In absolute terms, the difference was approximately 7 ng/mL (16.7 ng/mL vs. 23.3 ng/mL). With both treatment regimens, a significant increase in the concentration of 25(OH)D was observed (p<0.001), although these values were higher with the biweekly regimen than with the monthly regimen (in absolute terms, 39.5 ng/mL and 15.5 ng/mL, which meant in relative terms increases of 323% and 85% [p<0.0001]). The final concentration was clearly higher in patients receiving the biweekly regimen (56.2±18.5 ng/mL vs. 38.8±12.5 ng/mL, p<0.01).
The percentage of patients who reached 25(OH)D levels above 20 and 30 ng/mL was slightly higher with the biweekly treatment (100% and 92%, respectively) than with the monthly regimen (97% and 80%, respectively). However, the percentage of patients exceeding 60 ng/mL was higher in patients who received the biweekly regimen of calcifediol, 38%, compared to 6% in patients with the monthly regimen (p<0.01).
The concentration of iPTH decreased significantly after the administration of calcifediol with both treatment regimens. It is interesting to note that, despite the existence of differences in the values of 25(OH)D at the end of the study, the PTH values at that time were similar with the two treatment regimens (Table 2, Figure 1). The changes in the levels of iPTH in absolute terms (-19% vs. -20%) were also similar. Therefore, a clear discrepancy is observed in the behavior of 25(OH)D and PTH.
Table 2. Concentrations of 25(OH)D, iPTH, PINP and CTX before (baseline) and after at least one year of treatment (after treatment) with 0.266 mg of fortnightly (biweekly) or monthly (monthly dose) calcifediol. Expressed as mean (SD)
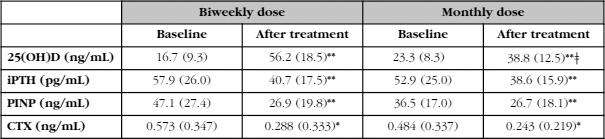
Differences between baseline values and after treatment: (*) p<0.01; (**) p<0.001.
Differences between the biweekly and monthly doses: (ǂ) p<0.01.
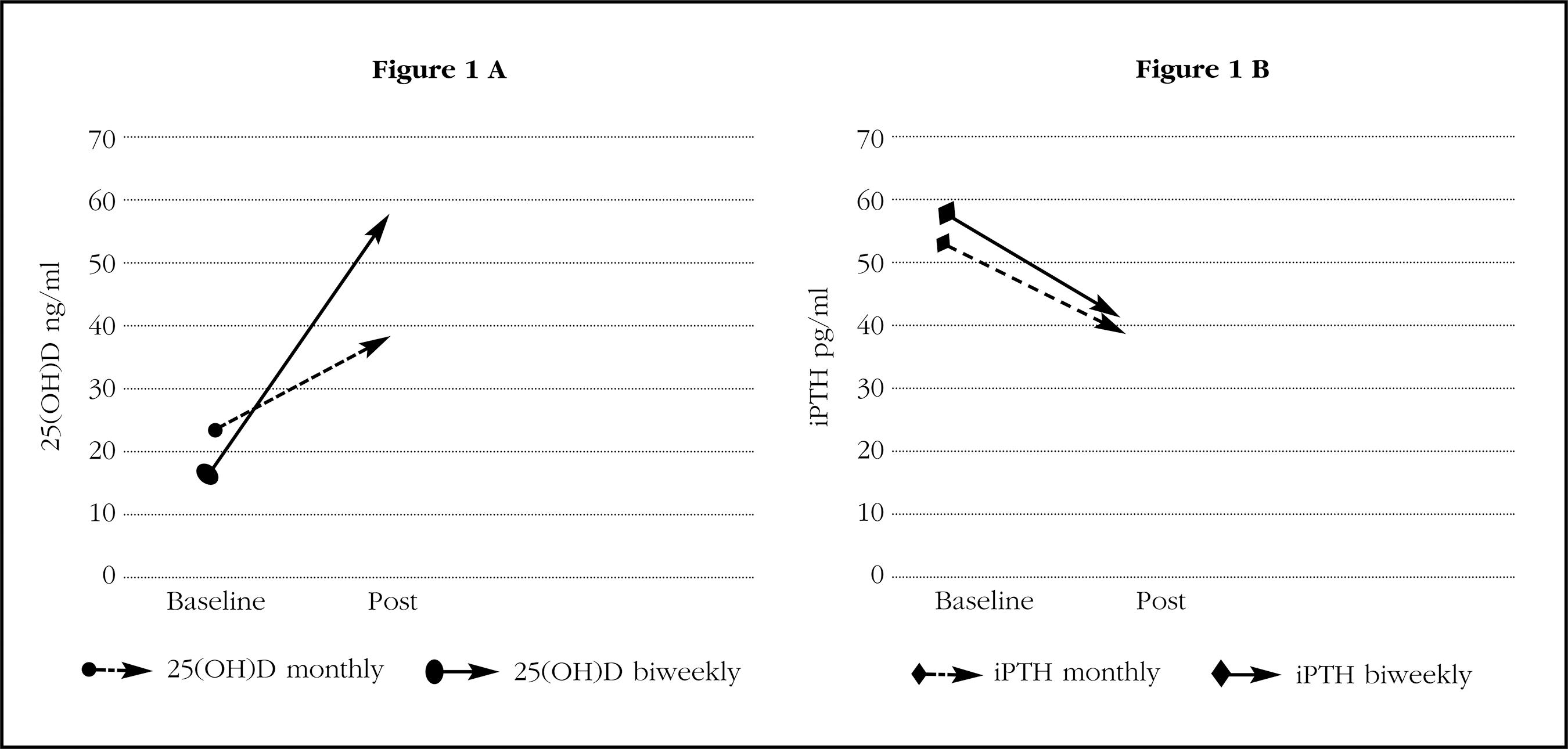
Figure 1. A) Average concentrations of 25(OH)D, before (baseline) and after at least one year of treatment (post) with 0.266 mg of monthly or fortnightly calcifediol. B) Mean concentrations of iPTH, before (baseline) and after at least one year of treatment (post) with 0.266 mg of calcifediol monthly or biweekly
All patients had normal calcium values at baseline and no case of hypercalcemia was detected with both treatment regimens.
The results of the remodeling markers (PINP and CTX) are also shown in table 2. As expected, both markers, PINP and CTX decreased significantly in patients treated with antiresorptives (p<0.0001), without these changes will be related to the dosage regimen of calcifediol (biweekly or monthly).
Finally, the changes in the levels of 25(OH)D, iPTH, PINP and CTX had no relationship with age, sex, weight, BMI or the month of the year in which the samples were obtained.
Discussion
In most of the clinical practice guidelines of osteoporosis, recommended serum levels of 25(OH)D are above 20 ng/mL, and even exceed 30 ng/mL9-11. In our study, the vast majority of patients who received monthly or biweekly doses of calcifediol for at least one year exceeded these figures. Specifically, the percentage of patients who reached levels 25(OH)D higher than 20 and 30 ng/mL was 100% and 92% with the biweekly regimen and 97% and 80% with the monthly, respectively.
As expected, the average concentrations of 25(OH)D reached by patients treated with calcifediol for at least one year were lower on the monthly than on the biweekly schedule. However, it can be concluded that both guidelines are effective for attaining metabolically sufficient 25(OH)D concentrations. A criterion conventionally used to assess the efficacy of serum levels of 25(OH)D involves its relationship with serum PTH, a negative relationship, since it inhibits the production of serum PTH. Since PTH is thought to be harmful to bone, its inhibition by vitamin D should be beneficial. Although there is no general agreement regarding the exact characteristics of this relationship, the most accepted idea is that as the levels of 25(OH)D rise, PTH decreases until it reaches a value above which the hormone is not suppressed more2,3,18-22. According to what we have been commenting, above this value of 25(OH)D, the beneficial effect does not continue to increase, as the PTH decreases. Although it is not known exactly at which concentration of 25(OH)D this value corresponds, it seems that there is agreement that it should be between 20 and 30 ng/mL, or close to these figures20-22. Our group studied more than 1,800 people (1,154 postmenopausal women and 657 men 50 years and older) and found that the threshold of 25(OH)D needed to prevent the increase of PTH (and the loss of bone mass) would be around 30 ng/mL22. The results of the present work point to an equivalent idea when showing that the concentration of iPTH decreases significantly after administering calcifediol without evident differences in the behavior of the hormone between the two treatment regimens. In fact, the reduction obtained with the biweekly schedule was 19% and 20% with the monthly pattern. So, from a certain value, vitamin D no longer exerts beneficial effects.
Studies concerning vitamin D doses and desirable 25(OH)D serum levels have usually focused on aspects related to their efficacy. The possible toxicity of vitamin D has traditionally been related to the development of hypercalcemia, which only occurs with high doses of vitamin D (several thousand-day units) and levels of 25(OH)D in ranges greater than 100 ng/mL3. It was thus considered a safe substance. However, over the last few years, data have been appearing suggesting that vitamin D could develop other pernicious effects, independent of hypercalcemia, with much lower levels. So, the idea that, in general terms, the relationship between the beneficial or harmful effects of vitamin D and its levels have a U-shaped relationship (that is, detrimental effects develop both with low levels and with high levels of the vitamin), with the particularity that in the latter case they would begin to settle down with levels of 25(OH)D very inferior to the 100 ng/mL previously commented, having indicated figures of around 50-60 ng/mL. Michaelson et al.23, for example, followed a cohort of 1,194 males observing a U-ratio between vitamin D concentration and total mortality. The morphology of the curve was also met in cancer mortality specifically. Durup et al.8 also described in 247,574 people a reverse association in the form of J between serum levels of 25(OH)D and mortality. Smith et al.24, in a study conducted with various doses of vitamin D supplements, observed that the effect of the same in the reductions also shows a U curve, both the relationship in terms of dose and levels of 25(OH)D analyzed. The various research studies carried out in this line do not coincide exactly with the values that could be preferable, but in general terms, reviews and consensus documents indicate that values greater than 50-60 ng/mL should be avoided5,7.
The results of our study show that while the figures reached with the monthly guideline are located in a safety zone (38.8±12.5 ng/mL), those obtained with the biweekly schedule do so in potentially harmful values (56.2±18.5 ng/mL), since the percentage of patients who exceeded 60 ng/mL among those who received the biweekly regimen was 38%, compared to 6% of those who received the monthly (p<0.01). Consequently, our work suggests that the therapeutic regimen with 0.266 mg of calcifediol monthly is the most appropriate. This does not mean, at all, that such a guideline should be considered rigidly, since in the achievement of one or other levels of 25(OH)D, factors of an individual nature are involved (for example, genetic) as circumstantial, which may advise modifying the recommended dose in a specific patient. Hence, the periodic measurement of serum 25(OH)D is recommended to check whether the regimen is adequate.
Regarding the behavior of remodeling markers, as expected, both markers, PINP and CTX, decreased significantly in patients treated with antiresorptives, both in those who received bisphosphonates and in those who received denosumab, without these changes being related to the dosage schedule of calcifediol (biweekly or monthly). This should not be interpreted in the sense that the provision of vitamin D does not influence the response to antiresorptive drugs. In a study carried out by our group in osteoporotic women receiving treatment with alendronate17, some of which were supplemented with 0.266 mg of calcifediol weekly for three months and others not, we found that the response was higher in the supplemented patients, especially when they started from a situation of basal hypovitaminosis (25(OH)D <20 ng/mL).
Among the limitations of our study, it should be noted that this is a retrospective observational study, so we cannot rule out the existence of certain biases (selection, allocation, etc.), as well as confounding factors. For example, in our study, the assignment of patients to one or another treatment regimen was not carried out randomly, but rather in accordance with the criteria of the physician in charge of the patient's care. It is not surprising, therefore, that patients who received the biweekly regimen were older and had baseline values lower than 25(OH)D than those who received the monthly regimen. However, one of the strengths of our study is that it has been carried out under conditions of normal clinical practice.
In conclusion, our results indicate that in patients with osteoporosis being treated for it, the monthly administration of 0.266 mg of calcifediol is, firstly, adequate to achieve effective levels of vitamin D, and secondly, sufficiently safe for avoid reaching potentially harmful levels of it, so it would be preferable to the biweekly schedule in routine clinical practice.
Bibliografía
1. Binkley N, Carter GD. Toward Clarity in Clinical Vitamin D Status Assessment: 25(OH)D Assay Standardization. Endocrinol Metab Clin North Am. 2017;46:885-99. [ Links ]
2. Dawson-Hughes B, Mithal A, Bonjour JP, Boonen S, Burckhardt P, Fuleihan GE, et al. IOF position statement vitamin D recommendations for older adults. Osteoporos Int. 2010;21:1151-4. [ Links ]
3. Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911-30. [ Links ]
4. Institute of Medicine (IOM). Dietary reference intakes for calcium and vitamin D. Washington, DC: National Academies Press, 2011. [ Links ]
5. Varsavsky M, Rozas Moreno P, Becerra Fernández A, Luque Fernández I, Quesada Gómez JM, et al.; en representación del Grupo de Trabajo de Osteoporosis y Metabolismo Mineral de la Sociedad Española de Endocrinología y Nutrición. Recomendaciones de Vitamina D para la población general. Endocrinol Diabetes Nutr. 2017;64 (Suppl 1):7-14. [ Links ]
6. Gómez de Tejada Romero MJ, Sosa Henríquez M, Del Pino Montes J, Jódar Gimeno E, Quesada Gómez JM, Cancelo Hidalgo MJ, et al. Documento de posición sobre las necesidades y niveles óptimos de Vitamina D. Sociedad Española de Investigación Ósea y Metabolismo Mineral (SEIOMM) y Sociedades afines. Rev Osteoporosis Metab Miner. 2011;3:53-64. [ Links ]
7. Zittermann A. The biphasic effect of vitamin d on the musculoskeletal and cardiovascular system. Int J Endocrinol. 2017;3206240. [ Links ]
8. Durup D, Jørgensen HL, Christensen J, Schwarz P, Heegaard AM, Lind B. A reverse J-shaped association of all-cause mortalitywith serum 25-hydroxyvitamin D in general practice: The CopDstudy. J Clin Endocrinol Metab. 2012;97:2644-52. [ Links ]
9. Cosman F, de Beur SJ, LeBoff MS, Lewiecki EM, Tanner B, Randall S, et al. Clinician’s Guide to Prevention and Treatment of Osteoporosis. Osteoporos Int. 2014;25: 2359-81. [ Links ]
10. González-Macías J, Del Pino-Montes J, Olmos JM, Nogués X; en nombre de la Comisión de Redacción de las Guías de Osteoporosis de la SEIOMM. Guías de práctica clínica en la osteoporosis postmenopáusica, glucocorticoidea y del varón. Sociedad Española de Investigación Ósea y del Metabolismo Mineral (3ª. Versión actualizada 2014). Rev Clin Esp. 2015;215:515-26. [ Links ]
11. Compston J, Cooper A, Cooper C, Gittoes N, Gregson C, Harvey N, et al; National Osteoporosis Guideline Group (NOGG). UK clinical guideline for the prevention and treatment of osteoporosis. Arch Osteoporos. 2017;12:43. [ Links ]
12. Díez-Pérez A, Olmos JM, Nogués X, Sosa M, Díaz-Curiel M, Pérez-Castrillón JL, et al. Risk factors for prediction of inadequate response to antiresorptives. J Bone Miner Res. 2012;27:817-24. [ Links ]
13. Institute of Medicine (US). Committee to review dietary reference intakes for vitamin d and calcium, dietary reference intakes for calcium and vitamin D, (2011). http://www.ncbi.nlm.nih.gov/books/NBK56070/. (Acceso el 10/02/2018). [ Links ]
14. Jetter A, Egli A, Dawson-Hughe B, Staehelin HB, Stoecklin E, Goessl R, et al. Pharmacokinetics of oral vitamin D(3) and calcifediol. Bone. 2014;59;14-9. [ Links ]
15. Shieh A, Ma C, Chun RF, Witzel S, Rafison B, Contreras HTM, et al. Effects of CholecalciferolvsCalcifediol on Total and Free 25-Hydroxyvitamin D and Parathyroid Hormone. J Clin Endocrinol Metab. 2017;102:1133-40. [ Links ]
16. Navarro-Valverde C, Sosa-Henríquez M, Alhambra-Expósito MR, Quesada-Gómez JM. Vitamin D3 and calcidiol are not equipotent. J Steroid Biochem Mol Biol. 2016;164:205-8. [ Links ]
17. Olmos JM, Hernández JL, Llorca J, Nan D, Valero C, González-Macías J. Effects of 25-Hydroxyvitamin D3 therapy on bone turnover markers and PTH levels in postmenopausal osteoporotic women treated with alendronate. J Clin Endocrinol Metab. 2012;97:4491-7. [ Links ]
18. Aloia JF, Talwar SA, Pollack S, Feuerman M, Yeh JK. Optimal vitamin D status and serum parathyroid hormone concentrations in African American women. Am J Clin Nutr. 2006;84:602-9. [ Links ]
19. Vieth R, Ladak Y, Walfish PG. Age-related changes in the 25-hydroxyvitamin D versus parathyroid hormone relationship suggest a different reason why older adults require more vitamin D. J Clin Endocrinol Metab. 2003;88:185-91. [ Links ]
20. Kuchuck NO, Pluijm SM, van Schoor NM, Looman CWN, Smit JH, Lips P. Relationships of serum 25-hydroxyvitamin D to bone mineral density and serum parathyroid hormone and markers of bone turnover in older persons. J Clin Endocrinol Metab. 2009;94:1244-50. [ Links ]
21. Nakamura K, Nashimoto M, Tsuchiya Y, Saito T, Nishiwaki T, Ueno K, et al. Threshold value of serum 25hydroxyvitamin D concentration in relation to elevated serum parathyroid hormone concentrations in elderly Japanese women. J Bone Miner Res. 2006;24:395-400. [ Links ]
22. Olmos JM, Hernández JL, García-Velasco P, Martínez J, Llorca J, González-Macías J. Serum 25-hydroxyvitamin D, parathyroid hormone, calcium intake, and bone mineral density in Spanish adults. Osteoporos Int. 2016;27:105-13. [ Links ]
23. Michaëlsson K, Baron JA, Snellman G, Gedeborg R, Byberg L, Sundström J, et al. Plasma vitamin D and mortality in older men: a community-based prospective cohort study. Am J Clin Nutr. 2010;92:841-8. [ Links ]
24. Smith LM, Gallagher JC, Suiter C. Medium doses of daily vitamin D decrease falls and higher doses of daily vitamin D3 increase falls: A randomized clinical trial. J Steroid Biochem Mol Biol. 2017;173:317-22. [ Links ]
Received: April 14, 2018; Accepted: June 25, 2018











 text in
text in 


