My SciELO
Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista de Osteoporosis y Metabolismo Mineral
On-line version ISSN 2173-2345Print version ISSN 1889-836X
Rev Osteoporos Metab Miner vol.10 n.4 Madrid Nov./Dec. 2018 Epub Apr 03, 2023
https://dx.doi.org/10.4321/s1889-836x2018000400008
Special Documents
Review of the scientific evidence regarding clinical use of the Trabecular Bone Score (TBS) SEIOMM official position (2018)
1Servicio de Reumatología - Unidad de Metabolismo Óseo - Hospitales Universitarios de la Esperanza y del Mar - Parque de Salud Mar - Unidad de Investigación en Fisiopatología Ósea y Articular (URFOA) - Instituto Hospital del Mar de Investigaciones Médicas (IMIM) - Barcelona (España)
2CETIR - Grupo Médico - Grupo Ascires - Barcelona (España)
3Servicio de Medicina Interna - Hospital Universitario Marqués de Valdecilla - Instituto de Investigación Sanitaria Valdecilla (IDIVAL) - Universidad de Cantabria - Santander (España)
4Departamento de Medicina - Universidad de Sevilla - Sevilla (España)
5Unidad de Gestión Clínica de Endocrinología y Nutrición - Hospital Universitario San Cecilio - Departamento de Medicina - Universidad de Granada - Instituto de Investigación Biosanitaria (IBS) - Granada (España)
Introduction
The incorporation of new technological applications in the medical field entails a prolonged period of evaluation of the scientific evidence generated in the clinical validation process.
Over the past 5 years, numerous publications, communications in congresses and meetings of scientific societies have been generated. The application of the Trabecular Bone Score (TBS) has also received the attention of the International Society for Clinical Densitometry (ISCD), which has integrated it into its official positions.
The concept of Evidence-Based Medicine (EBM) was developed by a group of internists and clinical epidemiologists led by Gordon Guyatt of McMaster University School of Medicine in Canada. The concept of EBM was defined by its creators as the conscious, explicit and judicious use of the best available clinical evidence to make decisions about the care of individual patients. In essence, EBM aims to have the best available scientific information, the evidence, to apply it to clinical practice.
In 2014, the Spanish Society of Bone Research and Mineral Metabolism (SEIOMM) began a project that facilitated its partners TBS software assessment, through a competitive call. The project ended in 2017. This application requires densitometry images with DXA (Dual X-ray Absorptiometry) of the lumbar spine, and by analyzing the image texture, offers information related to the microstructural quality of the trabecular bone. The project had the logistical support of Medimaps, a French developer, which distributed 20 TBS licenses among the partners that proposed their use in certain clinical and therapeutic settings.
Simultaneously, the diagnostic performance in predicting fractures in subjects with decreased bone density, the identification of those who have suffered bone fractures and the evaluation of this new parameter in patient follow-up was assessed.
In order for SEIOMM to achieve a global positioning that it can share with its partners, several experts of the Society have carried out a critical review of the existing scientific evidence on the clinical application of TBS, which is presented here.
Depending on the scientific rigor of the studies’ design, their quality is assessed using scales of hierarchical classification of the evidence, from which recommendations are established regarding the adoption of a specific medical procedure or health intervention. All of them have common features. In this case we have used the one used by the Scottish Intercollegiate Guidelines Network (SIGN), since the one proposed by the Agency of Evaluation of Medical Technology (Agència d'Avaluació de Tecnologia Mèdica -AATM-) of the Generalitat of Catalonia, which also takes into account the design of the studies, the specific assessment of their quality, requires a volume of scientific evidence created over a longer period of time that allows for producing more publications.
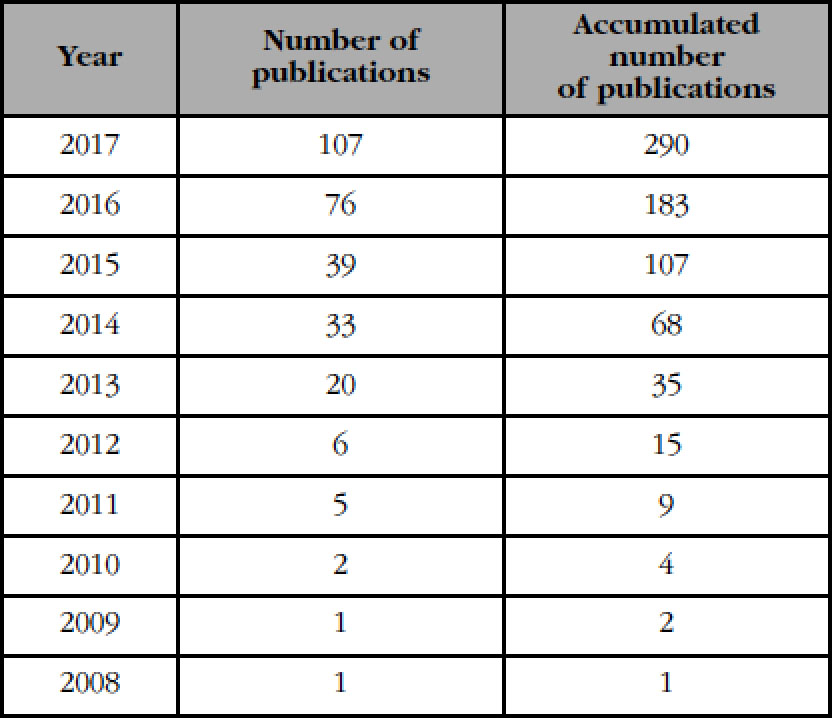
The first work describing the technique and its clinical use date back to 2009-2010. Not until 2013 was there a noticeable increase in the penetration of the new technique and the description of its results (Table 1).
Table 1 Number of publications describing the technique andits clinical use from 2008 to 2017 (provided by courtesy ofMedimaps)
Interest in this new application for evaluation of the DXA technique for microstructural quality estimation of trabecular bone has experienced an exponential increase, as can be seen in the publication quality graph (Figure 1).
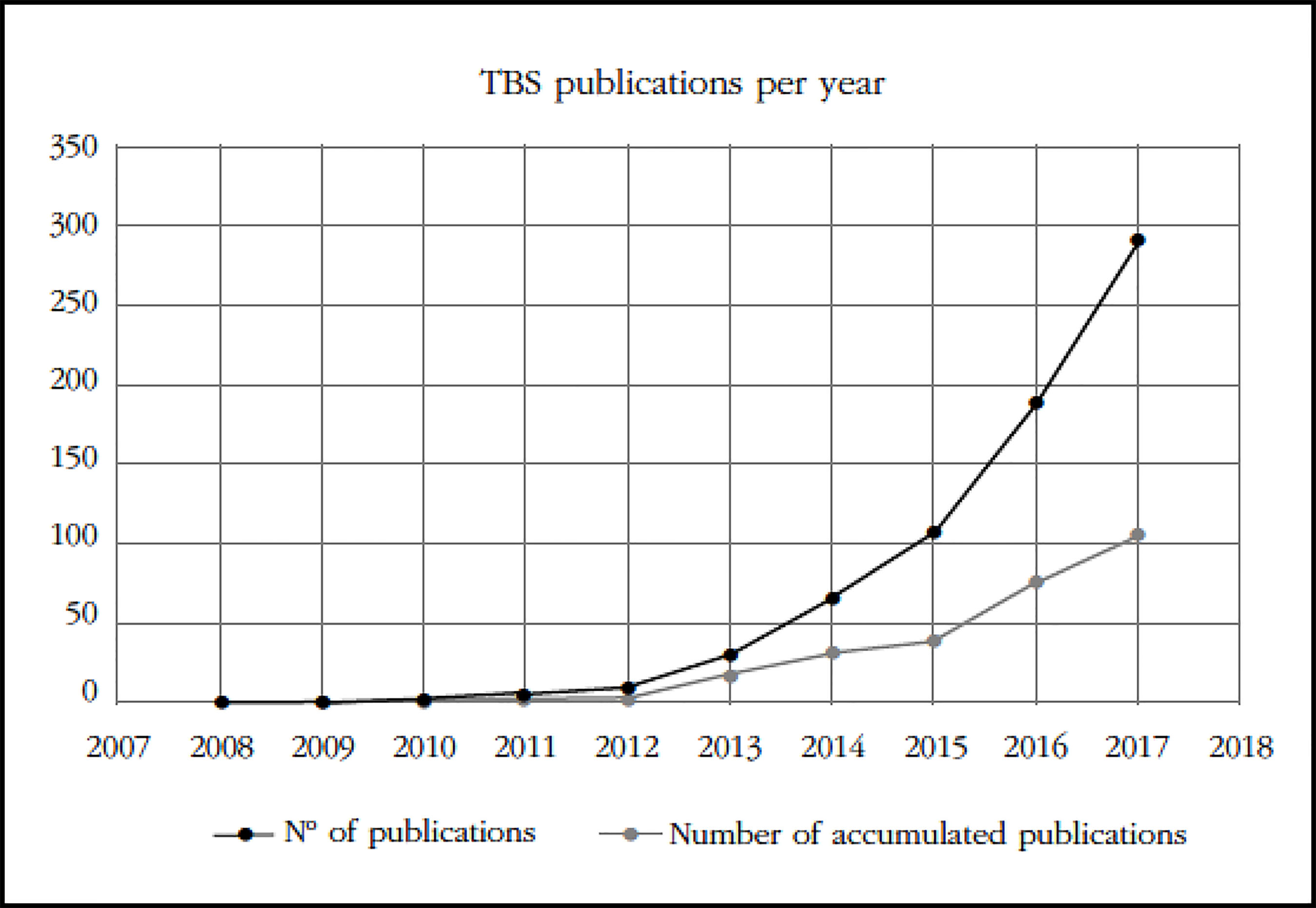
Figure 1 Number of presentations and publications on TBS presented in the period 2008-2017 (provided by courtesy of Medimaps)
The main international scientific institutions (the American Society for Bone and Mineral Research -The American Society for Bone and Mineral Research [ASBMR]-, the International Osteoporosis Foundation -International Osteoporosis Foundation [IOF]-, the ISCD) dedicated to the field of Metabolic osteopathies and especially the clinical management of osteoporosis have been the main destination of the presentations and publications on the TBS (Figure 2).
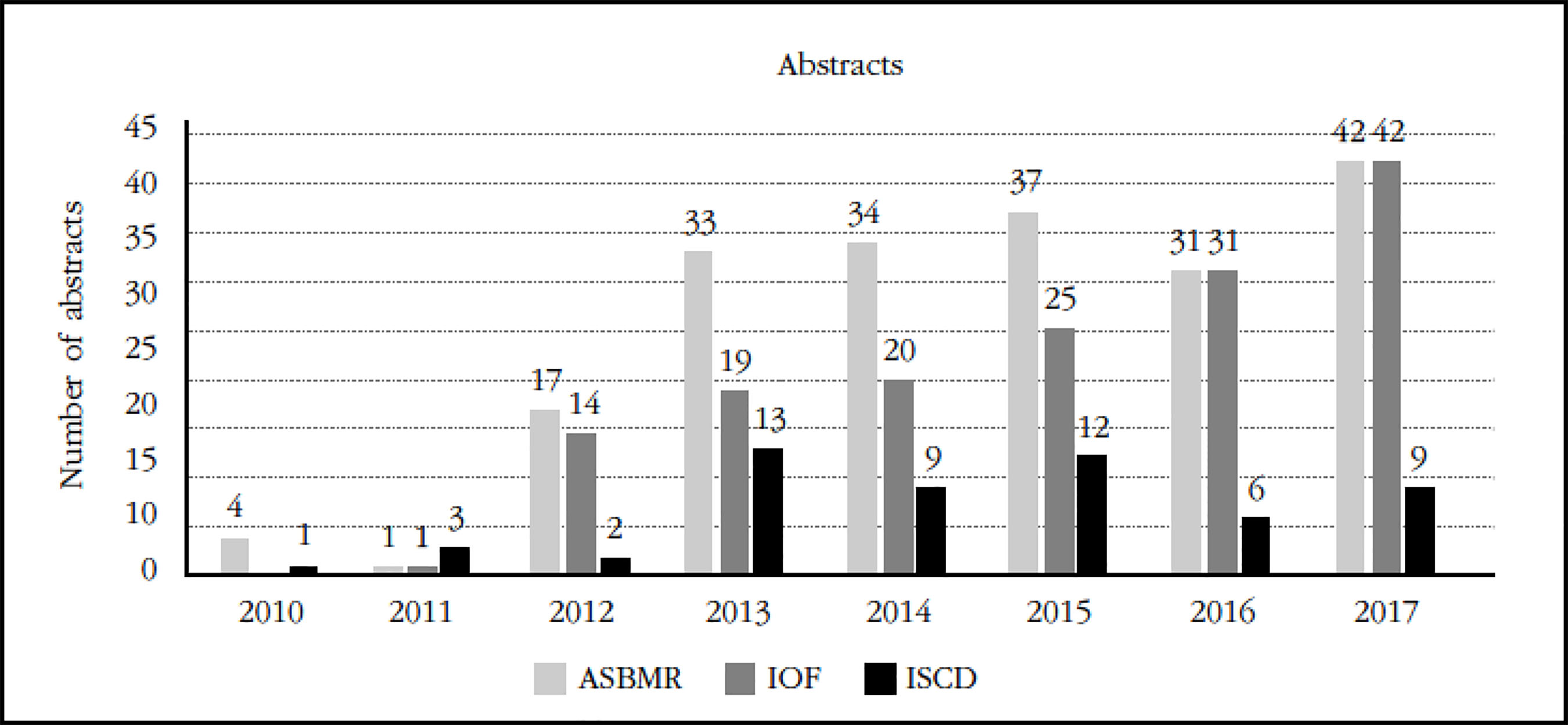
Figure 2 Abstracts on the TBS presented to congresses of the ASBMR, IOF, ISCD (provided by courtesy of Medimaps)
The experts' evaluation proposed by the SEIOMM has followed the methodological criteria of the SIGN scale (Table 2), which indicates the level of quality of the scientific evidence and the degree of recommendation that according to it is offered to the readers. A selection of the main publications related to the clinical aspects in which the TBS can influence has been carried out.
Table 2 Levels of evidence and degrees of recommendation of the Scottish Intercollegiate Guidelines Network (SIGN)
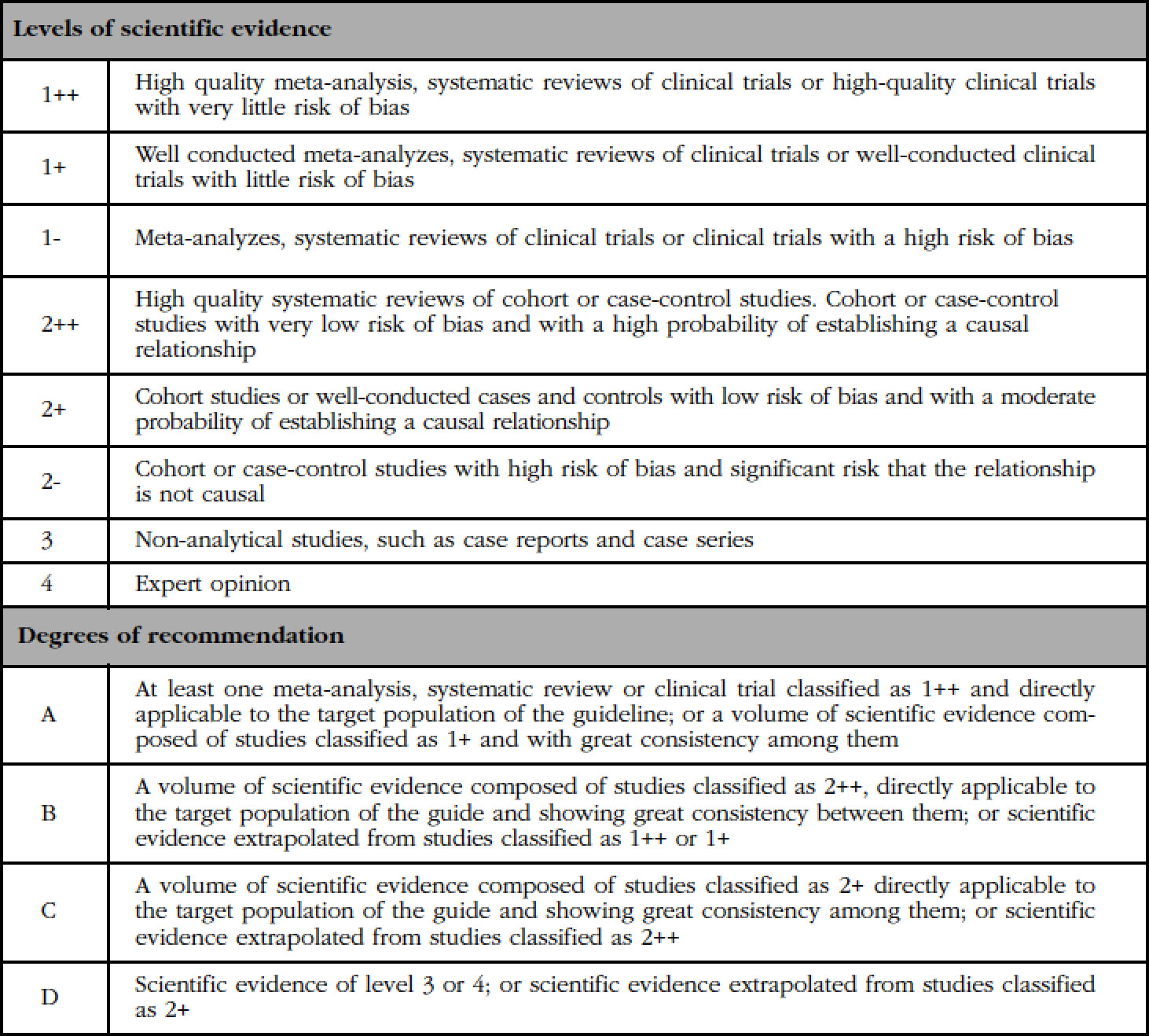
Studies classified as 1- and 2- should not be used in the process of making recommendations because of their high potential for bias.
The document divides the review process to address three major issues:
Can TBS be used to assess the risk of fracture in clinical practice?
Can TBS be used to monitor patients with osteoporosis?
In what diseases is TBS especially useful?
The SEIOMM experts who have carried out the review of the scientific evidence are Dr. María José Montoya, Dr. José Manuel Olmos Martínez and Dr. Manuel Muñoz, coordinated by Dr. Luis del Río.
Designated issues and reviewers
Reviewer: Dr. José Manuel Olmos Martínez
1. Question: Can TBS be used to assess the risk of fracture in clinical practice?
Proposal of statement 1: The TBS can be used to assess the risk of vertebral fracture, femur and global frailty in women and men from 50 years.
Proposal for statement 2: TBS can be used together with bone mineral density (BMD) to assess vertebral, femur and global fragility in men and women from 50 years of age.
Reviewer: Dr. Mª José Montoya
2. Question: Can TBS be used to monitor patients with osteoporosis?
Proposal for statement 1: The TBS can be used to evaluate changes over time.
Proposal for statement 2: The TBS can be used to assess the effects of treatment over time.
Reviewer: Dr. Manuel Muñoz
3. Question: In what diseases is TBS especially useful?
Proposal for statement 1: The TBS can be used to assess the risk of fracture in subjects with diabetes.
Proposal for statement 2: The TBS can be used to assess the risk of fracture in subjects treated with glucocorticoids.
Proposal for statement 3: The TBS can be used for the clinical orientation of subjects suffering from hypo and hyperparathyroidism.
Proposal for statement 4: TBS can be used for the diagnostic orientation of patients in the presence of osteoarthritis.
1. Question: Can TBS be used to assess the risk of fracture in clinical practice?
Proposal of statement 1: The TBS can be used to assess the risk of vertebral fracture, femur and global frailty in women and men from 50 years.
Summary: In 2013, Leslie et al.1 conducted a retrospective study of a cohort of 29,407 women over 49 years of age in which they assessed the relationships between TBS and the main clinical risk factors for osteoporosis. These authors, using linear regression and multiple regression models, demonstrated that the existence of a low TBS was associated with the recent use of glucocorticoids, a history of previous major fractures, rheumatoid arthritis, chronic obstructive pulmonary disease, high alcohol consumption and an index of high body mass. In contrast, recent therapy against osteoporosis was associated with a significantly lower probability of having a reduced TBS. Therefore, the authors concluded that TBS was strongly associated with many of the predictive risk factors for osteoporotic fractures, which in turn are incorporated into the WHO FRAX® tool. (The FRAX® tool includes the following clinical risk factors: body mass index (BMI), previous fracture, chronic obstructive pulmonary disease (smoking), use of glucocorticoids >90 days, rheumatoid arthritis, secondary osteoporosis and high alcohol consumption). More recently, McCloskey et al.2, after monitoring a cohort of 33,352 women aged 40-99 years from the Canadian province of Manitoba, found that TBS remained a statistically significant predictor of major osteoporotic fractures, excluding fracture. of hip (hazard ratio/standard deviation -HR/DE- =1.18 [95% CI: 1.12-1.24]), death (HR/SD=1.20 [95% CI: 1.14-1,26]) and hip fracture (HR/SD=1.23 [95% CI: 1.09-1.38]) after complete adjustment for the risk factors included in FRAX®. These authors3, in a meta-analysis in which they evaluated 17,809 women and men from 14 prospective cohorts, showed that, after adjusting for the absolute risk of fracture to 10 years provided by the FRAX® tool, TBS continued to act as a risk factor independent of fracture, both main and hip.
Proposal for statement 2: The TBS can be used together with the BMD by area (BMD) to assess vertebral, femur and global fragility in men and women from 50 years of age.
Summary: In a retrospective case-control study that assessed the diagnostic performance (sensitivity and specificity) of TBS, BMD and both techniques4, it was shown that the presence of low TBS and BMD was associated with fractures. in a more powerful way than when only the BMDa is decreased. Thus, the area under the curve (AUC) obtained from the ROC curves was in the first case (TBS and low BMD) 0.732 compared to 0.614 (p=0.005) when only the BMD was low, with the odds ratio (OR) of 2.49 (95% CI: 1.86-3.47) versus 1.54 (95% CI: 1.17-2.03), respectively. On the other hand, del Río et al.5 found that the combination of TBS and BMD in the lumbar spine improved the prediction of fracture risk in the upper third of the femur. These authors also found that, after adjusting for age, lumbar BMD and TBS maintained their ability to significantly discriminate transcervical fractures (OR=1.94 [95% CI: 1.35-2.79]; 71 [95% CI: 1.152.55]), respectively. On the other hand, Leib et al.6 have obtained consistent results in a larger cohort of Caucasian non-Hispanic American women (n=2,165). In fact, after adjusting for age, weight, BMD, smoking, and family and maternal fracture history, TBS remained a significant predictor of fracture, with an OR of 1.28 (95% CI: 1.13-1.46). The model that combines TBS and BMD increased the association with the fracture by 10%, as expressed by an increase in probabilities of 38% (OR=1.38 (95% CI: 1.23-1.55)). In another study carried out in a small number of women, the combination of TBS and lumbar spine BMD (OR=2.39 [95% CI: 1.70-3.37]) improved the prediction of fracture risk by 25%. Hans et al.7 showed that the combination of BMD measurement in any region of interest (lumbar spine, femoral neck or total hip) with TBS significantly improved the prediction of fractures compared to BMD or TBS alone ( p<0.0001). Briot et al.8 finally showed that, for the prediction of vertebral fractures, the combination of TBS and BMD of the lumbar spine increased performance in relation to the isolated use of BMD in the lumbar spine (Net Reclassification Improvement -NRI- =8.6%, p=0.046). Therefore, the determination of TBS has recently been incorporated into the factors used by the FRAX® tool to calculate the risk of osteoporotic fracture, which seems to improve the predictive capacity of this instrument for assessing the absolute risk of fracture9.
2. Question: Can TBS be used to monitor patients with osteoporosis?
Proposal for statement 1: The TBS can be used to evaluate changes over time.
Proposal for statement 2: TBS can be used to assess the effects of treatment over time.
After the review of the evidence the proposal of the statement 2: TBS does not improve the monitoring of BMD in the assessment of treatment effects over time.
Summary: For a measurement method to be useful in the follow-up of patients, it must be precise and changes influenced by a pathological situation or derived by a treatment must be equal to or greater than minimum significant change (MSC). Several studies have evaluated the accuracy of TBS measurements and have been compared to BMD measurements in the same DXA measurement systems. The first study concerning TBS accuracy was carried out by Hans et al.7 who evaluated 92 patients from the Manitoba study database, including women aged ≥ 50 years (51 performed on the same day and 41 remaining carried out after 28 days). The measurement’s precision was good with a coefficient of variation of 2.1%. Five other studies found similar results8,10-13. In general, TBS accuracy (1.1-2.1%) was comparable to the accuracy of BMD measurements (0.9-1.7%), and there were no significant differences between the different DXA devices. With a confidence interval of 95%, the MSC of TBS is 3.0-5.8%. All these studies included only women. In a more recent study by Krueger et al.14 a large number of men were included and similar results were found. Of 90 women and 90 men evaluated in a GE-Lunar iDXA by 3 different operators, the same day accuracy was 1.4% for TBS and 1.9% for BMD of the lumbar spine, without significant differences between sexes.
In addition to good accuracy, a useful measure in the follow-up of patients with treatment or derived from the pathological situation requires that the change be of sufficient magnitude to be detected. Several cross-sectional studies have shown a significant decrease in TBS with age.
In a study of 5,942 French Caucasian women11 a linear decrease of 14.5% was found in TBS between 45 and 85 years of age. 8.5% of this loss occurred after 65 years of age. Similarly, a 16% decrease in TBS was observed in 619 Caucasian women between 45 and 90 years old15. In a study of 3,069 Japanese women aged 45-80 years, a 19% decrease in TBS was detected16. In 518 African-American women aged 50-80 years, there was a less pronounced decrease in TBS of 4.6%17. The most important longitudinal study based on the Manitoba database sample size found a significant decrease of 0.31±0.06% per year in the TBS during an average follow-up of 3.7 years, similar to the decrease of 0.36±0.05% per year observed in the BMD of the lumbar spine in untreated patients18.
Currently, there are several types of effective and safe drugs for the treatment of osteoporosis and in the studies reviewed in preparation of this paper, one or more of these treatments were evaluated. The TBS has been analyzed in 12 studies in patients treated with bisphosphonates, in 5 of them with denosumab, in 7 with anabolic therapy (teriparatide), in 2 with vitamin D, and in 1 with testosterone. Bisphosphonate therapy was associated, in 8 of the studies, with a significantly higher TBS change compared to the untreated controls19-26. However, in 2 studies this fact could not be proven, but it should be noted that in one of them there were patients with osteoporosis induced by glucocorticoids31, and the other was carried in patients undergoing recent liver transplantation24. In a retrospective cohort study, broad in terms of the number of subjects, carried out by Krieg et al., changes in TBS were compared in 534 postmenopausal women treated (with compliance greater than 75%) or with bisphosphonates (86%), raloxifene (10%) or calcitonin (4%); compared to 1,150 untreated women. During the follow-up, with an average of 3.7 years, TBS reportedly increase in treated women by 0.2%/year, while it decreased in untreated women by 0.3%/year (changes that were statistically significant compared to the initial value)28. One of the most relevant studies that analyzes the effect of bisphosphonates on TBS was carried out by Leslie et al., in a retrospective cohort. This work is important because of the high number of subjects included (5,083 women treated, mostly with bisphosphonates -80%-, and 3,961 women without antiosteoporotic treatment) and for the long period of follow-up (average of 4.1 years). These authors found greater gains in TBS in women with greater adherence to the medication for osteoporosis (-1.2% change in TBS for untreated patients, versus +0.8% change for treated patients, with high adherence index treatment (>0.8, p for the trend <0.001) .In spite of this, and taking into account that the main objective of this study was to investigate whether the change in TBS affected the risk of fracture independently, he was able to verify this fact, concluding that the change in TBS is not a useful indicator of fracture risk23.
Changes in TBS with bisphosphonates are generally of small magnitude. A clinical trial that evaluated the effect of zoledronic acid (at doses higher than those used in osteoporotic disease) vs. placebo in premenopausal women with breast cancer, has indicated greater increases in TBS at 2 years (of 2.41%, versus -2.16% of the placebo group)22.
Anabolic medication was also associated with significant increases in TBS consistently in 4 studies19,20,31,34 and in some cases this effect is described as early as 3 months after teriparatide treatment has commenced27. These changes are of greater magnitude than those indicated for bisphosphonates. The increase in TBS has been demonstrated, both in an open longitudinal study of patients with primary osteoporosis13 and in a subanalysis of a clinical trial of patients with osteoporosis due to corticosteroids, in which the effect of teriparatide vs. alendronate was compared20. In the latter, a greater TBS increase is also shown in the group with anabolic therapy, reaching 3.6% at 36 months against the baseline value, in the teriparatide branch. In the DATA-Switch clinical trial, Tsai et al. reported that after 48 months of treatment, TBS increased by an average value of 5.1, 3.6, and 6.1% with sequential teriparatide therapy, denosumab, denosumab-teriparatide or the combination of both, respectively33. Similarly, although an open two-year study, Senn et al., compared changes in TBS in 65 patients treated with teriparatide vs. 122 treated with ibandronate, showed that patients treated with teriparatide had a 4.3% increase in the TBS (p<0.001 compared to the initial value) and significantly higher than that observed in the group treated with ibandronate (0.3%)13. On the other hand, only one study, with low statistical power (only 14 subjects), that assessed TBS in patients with atypical fractures and treatment with teriparatide, did not observe significant changes in this index34.
Other research studies of denosumab antiresorptive therapy also reported significant improvement in TBS19,29,32,33. Recently, McClung et al. compared TBS and BMD in 157 postmenopausal women treated with denosumab versus 128 women with placebo in a subanalysis of patients in the FREEDOM clinical trial. In the denosumab group, progressive increases were seen from baseline at 12, 24 and 36 months for TBS (1.4, 1.9 and 2.4%, respectively). The percentage changes in TBS were statistically significant compared to baseline and placebo, in addition to being, to a large extent, independent of BMD and changes in BMD, induced both by time and by the effect of treatment32. Increases in TBS of greater magnitude have also been reported in postmenopausal women with osteopororis corticoidea after one year of treatment with denosumab, reaching an average TBS increase of 5%26.
Interestingly, TBS changes have also been used to evaluate the effect of switching from one treatment to another. In this sense, Ebina et al.,20 in a nonrandomized observational study, found in women with rheumatoid arthritis and corticosteroid treatment that the change in the treatment from bisphosphonates to teriparatide produced an increase in TBS greater than the change to denosumab (2.1. vs. -0.7%). In addition, the change to teriparatide attained a significantly higher elevation in TBS than that obtained in the group that continued with bisphosphonates (2.1 vs. -1.8%)20. Similarly, Tsai et al. found, after 48 months of follow-up in a subanalysis of a clinical trial, that the change from teriparatide to denosumab increased the TBS with a greater magnitude than did the change from denosumab to teriparatide (5.8 vs. 3.6%, respectively)33.
Changes in TBS induced in patients by calcium and vitamin D treatment were less consistent. In a study carried out in 87 patients followed over 24 months, TBS results showed higher values compared to patients who had not received treatment19. However, these results were not replicated in a clinical trial that compared the effects on TBS of low dose and high dose of cholecalciferol versus placebo, after 12 months, in 230 postmenopausal women21.
The effect of testosterone treatment on TBS has only been evaluated in a study carried out in a small group of male patients with testosterone deficiency and with substitution treatment, showing a significant increase of 5% at 24 months19.
In most of the studies reviewed, the relationship of TBS and BMD values was reportedly low, and after treatment with the different antiosteoporotic drugs, the changes induced in BMD were clearly superior to those obtained with TBS. The relationship between both parameters is lost. This situation is especially noteworthy in the treatment with bisphosphonates.
Much of the scientific evidence reviewed shows that TBS provides a complementary and largely independent value to BMD measurements, so it is not expected that the response to bony changes by an antiosteoporotic treatment will be similar. Bone changes with TBS are especially modest in the treatment with bisphosphonates, in many cases remaining below the CMS. This has led the International Society of Clinical Densitometry (ISCD) not to recommend TBS in the monitoring of the response to the treatment of osteoporosis with bisphosphonates35,36.
3. Question: In what diseases is TBS especially useful?
Proposal for statement 1: TBS can be used to assess the risk of fracture in subjects with diabetes.
Summary: Patients with type 2 diabetes (DM2) have paradoxically a higher BMD and an increased risk of fragility fractures. In 8 studies it has been shown that, although BMD tends to be higher in type 2 diabetics than in non-diabetics, TBS tends to be lower in type 2 diabetics than in non-diabetics.
A cross-sectional case-control study conducted by Dhaliwal et al.37 compared 57 women with type 2 diabetes with 43 women without it. TBS was lower and BMD increased among diabetics (p =
0.001 and 0.01, respectively). On the other hand, the TBS was lower (p=0.01) and the BMD did not show significant differences in diabetics with poor glycemic control compared to those with good glycemic control (previous A1c <7.5%). These data were confirmed in a larger study38 that included 1,229 men and 1,529 postmenopausal women older than 50 years of the Korean Ansung cohort. TBS in the lumbar spine was significantly lower in women and men with diabetes than in non-diabetic women and men, while BMD in the lumbar spine was significantly higher in subjects with diabetes. Other recent case-control studies confirmed these findings in 131 diabetic patients and 265 controls39 and in 88 diabetic patients and 88 controls40. Holloway et al.41 observed the same trend in subjects with normoglycaemia, patients with high fasting fasting glucose (GAB) and diabetic patients. Diabetic or high GBA patients had higher BMD in the lumbar spine and lower TBS than patients with normoglycemia41.
Iki et al.42 observed a significantly higher BMD in men with diabetes compared to controls but did not observe significant differences in TBS. Fasting blood glucose levels, HbA1c and HOMA-IR (homeostasis model assessment index) correlated significantly inversely with TBS after adjusting for age, BMI and BMD. The multivariate linear regression analysis revealed that the glycemic indexes (GBA and HbA1c) were significantly associated with an increase in BMD and a decreased TBS, and that the evaluation of insulin resistance by the HOMA model was only associated with the TBS These associations were not modified after further adjustment for markers of bone turnover and pentosidine levels. These data were confirmed in a Korean population study (894 controls and 325 diabetic patients) where TBS was also negatively correlated with GBA, HbA1c and HOMA-IR.
Leslie et al.43 included in a study 29,407 Canadian women aged 50 years or older with reference DXA scans, of which 2,356 had been diagnosed with diabetes. After adjusting for clinical risk factors, it was found that diabetic women were more likely to be in the lower tertile of lumbar TBS, but were less likely to be in the lower tertiles of BMD of the lumbar spine, neck of the femur or total femoral area. The TBS values were a predictor of incident fractures independent of BMD.
In addition, Zhukouskaya et al.44 evaluated how the TBS and BMD variables could be useful to identify vertebral fractures (FxV) in a cohort of 99 patients (postmenopausal women) with wellcompensated type 2 diabetes (T2D). They compared these patients with T2D with 107 control subjects without T2D. They found that patients with DM2 had a higher prevalence of FxV compared to controls (34.3 vs. 18.7%, p=0.01). TBS was not different between well compensated type 2 diabetic patients and controls, but interestingly, TBS was decreased in patients with DM2 and fractures.
On the other hand, Bonaccorci et al.40 compared possible predictors of fractures in a group of 80 women with DM2 and 88 controls, and showed that TBS (AUC=0.71) and adjusted FRAX® for TBS (AUC=0,74) were the only statistically significant parameters in the diabetic group, unlike BMD and structural analysis of the femur. Finally, Choi et al.45, in a study conducted with 169 Korean postmenopausal women with DM2, found a significantly lower TBS (p=0.008) and a FRAX® score adjusted for the highest TBS (p=0.019) in the group. with FxV compared to the group without FxV. In contrast, there were no significant differences in BMD and original FRAX® scores between the 2 groups. The TBS (OR=1.8 [95% CI: 1.1-2.7], p=0.011) and the FRAX® score adjusted by the TBS (OR=2.0 [95% CI: 1.1-3.5], p=0.020) showed statistically significant ORs for FxV. The TBS and the FRAX® adjusted by TBS could be supplementary tools to discriminate osteoporotic fractures in DM2.
Proposal for statement 2: The TBS could be useful to evaluate the risk of fracture in subjects treated with glucocorticoids or endogenous hypercortisolism.
-Evidence grade, 2+.
Summary: Glucocorticoids (GC) produce rapid bone loss and an increased risk of fracture that can not be completely explained by changes in BMD. Leslie et al.46 investigated the clinical risk factors associated with TBS. Among 29,407 women in the Manitoba cohort with DXA scans of the lumbar spine, 1,213 had a history of recent use of GC. They found that the probability of a reduced TBS value is increased in subjects with recent GC use after adjusting for BMD (OR=1.67 [95% CI: 1.40-1.99]). On the other hand, Leib et al. and Paggiosi et al.47,48 showed that TBS decreases in subjects treated with glucocorticoids and that TBS is more sensitive than BMD in these subjects. In their study, Paggiosi et al.48, who evaluated 484 women (mean age 67±7.5 years) of whom 64 had taken prednisolone (mean dose of 7.2±3.2 mg/day, mean duration of 9.2 ±10.8 years), found that subjects with GC had a significant decrease in TBS compared to women without prior treatment with GC, and there were no differences in BMD of the lumbar spine. These results were corroborated in a larger-scale study by Leib et al.47. This study involved 1,520 men and women aged 40 years or older. Among them, 416 subjects who received GC (dose ≥ 5 mg/day, for ≥ 3 months) were compared with 1,104 control subjects adjusted for similar sex, age and BMI. The authors demonstrated a significant decrease in TBS (p<0.001) compared to controls, whereas no change was observed in the BMD of the lumbar spine (p=0.88). In addition, they observed more pronounced decreases in TBS in males compared to females. Finally, they observed that this alteration of the TBS was even more pronounced when the subjects with GC and fracture were compared with the subjects with GC without fracture (p<0.01), or when compared with the controls (p<0.001). This study showed that TBS was associated with the presence of a fracture with an OR of 1.51 [95% CI: 1.23-1.86] per DE decrease in TBS and an AUC of 0.648 [95% CI: 0.599-0.693]. A recent small study by Chuang et al.49 confirmed these trends in 30 patients who received GC therapy for 24 months and in 16 without it. The results showed a significant decrease in the percentage change in the TBS for the lumbar spine and a greater probability of fracture estimated by FRAX® adjusted by the TBS.
One of the endogenous forms of glucocorticoid-induced osteoporosis (OIG) is the presence of adrenal incidentaloma (IS), which can induce subclinical hypercortisolism and increase the risk of fracture. In a cohort of 102 patients50, the authors established that subjects with SI had significantly lower TBS values than controls. It is noteworthy that patients with subclinical hypercortisolism (n=34) exhibited significantly lower TBS than those without subclinical hypercortisolism, expressed by a Z-score of TBS of -3.18±1.21 vs. -1.70±1.54 (p<0.0001), despite having a Z-score of normal BMD in the spine and femur. Finally, lumbar TBS was a predictor of incident fractures in an average of 40 months of follow-up, regardless of the patient's age, BMI and BMD of the lumbar spine. However, Belaya et al.51 found in a population of 182 patients with subclinical hypercortisolism that only the level of free cortisol in 24 h urine (24 hUFC) was the only predictor of fracture. These authors observed low TBS values in their population (average Z-score of the TBS= -1.86), while the decrease in BMD was lower than the average Zscore of the BMD= -1,60).
Proposal for statement 3: TBS may be useful in the clinical evaluation of patients with primary hyperparathyroidism.
-Evidence grade, 2+.
Summary: In primary hyperparathyroidism (PHPT), vertebral fractures (FxV) occur independently of BMD and may depend on the decrease in bone quality.
In their cross-sectional study, Romagnoli et al.12 observed a significantly lower TBS in 73 postmenopausal women with primary hyperparathyroidism (29 of them with a documented vertebral fracture) than in 74 controls of similar age. In addition, the presence of vertebral fractures was associated independently with the reduction of TBS (OR=0.003 [95% CI: 0-0.534], p=0.028). In a study that included both transverse and longitudinal components, Eller-Vainicher et al.52 compared 92 patients with primary hyperparathyroidism (74 of them were postmenopausal women and 18 were men older than 50 years) with the results of 98 controls recruited simultaneously in the clinic. In agreement with the previous study, TBS was lower in patients with primary hyperparathyroidism than in controls, and was significantly associated with vertebral fracture, even after adjustment for age, sex, BMI and BMD of the lumbar spine (adjusted OR=1,4 [95% CI: 1.1-1.9]). In the longitudinal phase of the study, 20 patients with primary hyperparathyroidism who underwent an effective parathyroidectomy were compared at 24 months of follow-up with 10 patients treated conservatively. In the surgery group, the average TBS score increased by 47% (p<0.01). In patients followed conservatively, TBS decreased significantly compared to non-fractured patients (p<0.048).
Finally, Silva et al.53 evaluated the relationship between TBS, high resolution peripheral quantitative computed tomography (HR-pQCT) and bone resistance (by finite element analysis) in distal radius and tibia in 22 postmenopausal women with mild primary hyperparathyroidism. They found that TBS was correlated with complete bone strength and all HR-pQCT indices, except for trabecular thickness and trabecular stiffness in the radius, whereas TBS was correlated with volumetric densities, cortical thickness, trabecular bone volume and the complete bone resistance of the tibia. The conclusion was that the TBS is a promising diagnostic tool in the clinical evaluation of the trabecular microstructure in those patients who suffer a milder form of primary hyperparathyroidism.
In patients with asymptomatic PHPT, Diaz-Soto et al.54 did not find significant differences in the TBS when comparing normocalcemic vs. hypercalcemic patients. Cipriani et al.55 investigated skeletal changes after the restoration of the euparatiroid state, and, unlike Rolighed et al.56, found no significant changes in TBS after parathyroidectomy in patients with PHPT. However, they found a significant increase in TBS after 18 months of treatment with recombinant parathormone (rhPTH) in hypoparathyroid patients.
Proposal for statement 4: TBS could be useful to assess bone fragility in patients with severe osteoarthritis.
-Evidence grade, 2+.
Summary: Lumbar osteoarthritis overestimates bone density measured by DXA.
In these studies, the impact of osteoarthritis of the lumbar spine on the TBS result was assessed based on a French cohort of 390 women aged 50 or older11 and a part of the OPUS cohort that included 727 postmenopausal women of 55 years of age or more57. In the study by Dufour et al.11, the presence of osteoarthritis was evaluated using the ISCD definition (a difference of more than 1 SD in the T-score between two adjacent vertebrae). In the study by Kolta et al.57, they used the Kellgren and Lawrence (KL) classification based on radiographs of the lateral lumbar spine. In both studies significant differences were observed between those with and without osteoarthritis in the bone mineral density measured by DXA. In the study by Kolta et al., The increase in BMD correlated with the severity of osteoarthritis (KL scale). However, the TBS values were not influenced by the presence of osteoarthritis in both studies11,57.
Review of the scientific evidence on the clinical use of TBS: Official positions of the SEIOMM
Summary
-
1. Question: Can TBS be used to assess the risk of fracture in clinical practice?
-
Question: Can TBS be used to monitor patients with osteoporosis?
-
3. Question: In what diseases is TBS especially useful?
TBS can be used to assess the risk of fracture in subjects with diabetes.
TBS can be used to assess the risk of fracture in subjects treated with glucocorticoids.
TBS can be used for the clinical orientation of subjects suffering from hypo and hyperparathyroidism.
-
TBS can be used for the diagnostic orientation of patients in the presence of osteoarthritis.
Acknowledgments
To the collaborators of Medimaps: Renaud Winzenrieth, Laureen Ferchaud, Marie-Emilie Mathieu, Doris Tran and Celine Gerard, for contributing to start the project and to proceed, for giving financial support, even when compensation was not clear; in short, for all your effort. Also our thanks to Didier Hans, director of Medimaps.
REFERENCES
1 Leslie WD, Krieg MA, Hans D; Manitoba Bone Density Program. Clinical factors associated with trabecular bone score. J Clin Densitom. 2013;16(3):374-9. [ Links ]
2 McCloskey EV, Odén A, Harvey NC, Leslie WD, Hans D, Johansson H, Kanis JA. Adjusting fracture probability by trabecular bone score. Calcif Tissue Int. 2015;96(6): 500-9. [ Links ]
3 McCloskey EV, Odén A, Harvey NC, Leslie WD, Hans D, Johansson H, et al. A meta-analysis of trabecular bone score in fracture risk prediction and its interaction with FRAX. J Bone Miner Res. 2016;31(5):940-8. [ Links ]
4 Winzenrieth R, Dufour R, Pothuaud L, Hans D. A retrospective case-control study assessing the role of trabecular bone score in postmenopausal Caucasian women with osteopenia: analyzing the odds of vertebral fracture. Calcif Tissue Int. 2010;86(2):104-9. [ Links ]
5 Del Rio LM, Winzenrieth R, Cormier C, Di Gregorio S. Is bone microarchitecture status of the lumbar spine assessed by TBS related to femoral neck fracture? A Spanish case-control study. Osteoporos Int. 2013;24(3):991-8. [ Links ]
6 Leib E, Winzenrieth R, Lamy O, Hans D. Comparing bone microarchitecture by trabecular bone score (TBS) in Caucasian American women with and without osteoporotic fractures. Calcif Tissue Int. 2014;95(3):201-8. [ Links ]
7 Hans D, Goertzen AL, Krieg MA, Leslie WD. Bone microarchitecture assessed by TBS predicts osteoporotic fractures independent of bone density: the Manitoba study. J Bone Miner Res. 2011;26(11):2762-9. [ Links ]
8 Briot K, Paternotte S, Kolta S, Eastell R, Reid DM, Felsenberg D, et al. Added value of trabecular bone score to bone mineral density for prediction of osteoporotic fractures in postmenopausal women: the OPUS study. Bone. 2013;57(1):232-6. [ Links ]
9 Martineau P, Leslie WD, Johansson H, Oden A, McCloskey EV, Hans D, et al. Clinical utility of using lumbar spine Trabecular Bone Score to adjust fracture probability: The Manitoba BMD cohort. J Bone Miner Res. 2017;32:1568-74. [ Links ]
10 Iki M, Tamaki J, Kadowaki E, Sato Y, Dongmei N, Winzenrieth R, et al. Trabecular bone score (TBS) predicts vertebral fractures in Japanese women over 10 years independently of bone density and prevalent vertebral deformity: the Japanese Population-Based Osteoporosis (JPOS) cohort study. J Bone Miner Res. 2014;29(2):399-407. [ Links ]
11 Dufour R, Winzenrieth R, Heraud A, Hans D, Mehsen N. Generation and validation of a normative, age-specific reference curve for lumbar spine trabecular bone score (TBS) in French women. Osteoporos Int. 2013;24(11): 2837-46. [ Links ]
12 Romagnoli E, Cipriani C, Nofroni I, Castro C, Angelozzi M, Scarpiello A, et al. “Trabecular Bone Score'' (TBS): an indirect measure of bone micro-architecture in postmenopausal patients with primary hyperparathyroidism. Bone. 2013;53(1):154-9. [ Links ]
13 Senn C, Günther B, Popp AW, Perrelet R, Hans D, Lippuner K. Comparative effects of teriparatide and iban-dronate on spine bone mineral density (BMD) and microarchitecture (TBS) in postmenopausal women with osteoporosis: a 2-year open-label study. Osteoporos Int. 2014;25(7):1945-51. [ Links ]
14 Krueger D, Libber J, Binkley N. Spine trabecular bone score precision, a comparison between GE Lunar standard and high-resolution densitometers. J Clin Densitom. 2015;18(2):226-32. [ Links ]
15 Simonelli C, Leib E, Mossman N, Winzenrieth R, Hans D, McClung M. Creation of an age-adjusted, dual-energy X-ray absorptiometry-derived trabecular bone score curve for the lumbar spine in non-Hispanic US white women. J Clin Densitom. 2014;17(2):314-9. [ Links ]
16 Iki M, Tamaki J, Sato Y, Winzenrieth R, Kagamimori S, Kagawa Y, et al. Age-related normative values of trabecular bone score (TBS) for Japanese women: the Japanese Population-based Osteoporosis (JPOS) study. Osteoporos Int. 2015;26(1):245-52. [ Links ]
17 Aloia JF, Mikhail M, Usera G, Dhaliwal R, Islam S. Trabecular bone score (TBS) in postmenopausal African American women. Osteoporos Int. 2015;26(3):1155-61. [ Links ]
18 Krieg MA, Aubry-Rozier B, Hans D, Leslie WD. Effects of anti-resorptive agents on trabecular bone score (TBS) in older women. Osteoporos Int. 2013;24(3):1073-8. [ Links ]
19 Di Gregorio S, Del Rio L, Rodriguez-Tolra J, Bonel E, Garcia M, Winzenrieth R. Comparison between different bone treatments on areal bone mineral density (aBMD) and bone microarchitectural texture as assessed by the trabecular bone score (TBS). Bone. 2015;75: 138-43. [ Links ]
20 Ebina K, Hirao M, Hashimoto J, Hagihara K, Kashii M, Kitaguchi K, et al. Assessment of the effects of switching oral bisphosphonates to denosumab or daily teriparati-de in patients with rheumatoid arthritis. J Bone Miner Metab. 2018;36(4):478-87. [ Links ]
21 Hansen KE, Johnson RE, Chambers KR, Johnson MG, Lemon CC, Vo TN, et al. Treatment of vitamin d insufficiency in postmenopausal women: A Randomized Clinical Trial. JAMA. 2015;175(10):1612-21. [ Links ]
22 Kalder M, Kyvernitakis I, Albert U S, Baier-Ebert M, Hadji P. Effects of zoledronic acid versus placebo on bone mineral density and bone texture analysis assessed by the trabecular bone score in premenopausal women with breast cancer treatment-induced bone loss: results of the ProBONE II substudy. Osteoporos Int. 2015;26(1):353-60. [ Links ]
23 Leslie WD, Majumdar SR, Morin SN, Hans D, Lix LM. Change in Trabecular Bone Score (TBS) with antiresorptive therapy does not predict fracture in women: The Manitoba BMD Cohort. J Bone Miner Res. 2017; 32(3);618-23. [ Links ]
24 Librizzi MS, Guadalix S, Martinez-Diaz Guerra G, Allo G, Lora D, Jimenez C, et al. Trabecular bone score in patients with liver transplants after 1 year of risedrona-te treatment. Transplant Int. 2016:29(3):331-7. [ Links ]
25 Rodríguez M, Pineda M, Servitja S, Garcia N, Martos T, Tusquets I, et al. TBS and BMD at the end of AI-the-rapy: A prospective study of the B-ABLE cohort. Bone. 2016;92:1-8. [ Links ]
26 McClung MR, Lippuner K, Brandi ML, Zanchetta JR, Bone HG, Chapurlat R, et al. 2017. Effect of denosumab on trabecular bone score in postmenopausal women with osteoporosis. Osteoporos Int. 2017; 28(10):2967-73. [ Links ]
27 Miyaoka D, Imanishi Y, Ohara M, Hayashi N, Nagata Y, Yamada S, et al. Effects of teriparatide and sequential minodronate on lumbar spine bone mineral density and microarchitecture in osteoporosis. Calcif Tissue Int. 2017;101(4):396-403. [ Links ]
28 Muschitz C, Kocijan R, Pahr D, Patsch JM, Amrein K, Misof BM, et al. Ibandronate increases sclerostin levels and bone strength in male patients with idiopathic osteoporosis. Calcif Tissue Int. 2015;96(6):477-89. [ Links ]
29 Petranova T, Sheytanov I, Monov S, Nestorova R, Rashkov R. Denosumab improves bone mineral density and microarchitecture and reduces bone pain in women with osteoporosis with and without glucocorticoid treatment. Biotechnol Biotechnol Equip. 2014; 28(6):1127-37. [ Links ]
30 Popp AW, Guler S, Lamy O, Senn C, Buffat H, Perrelet R, et al. Effects of zoledronate versus placebo on spine bone mineral density and microarchitecture assessed by the trabecular bone score in postmenopausal women with osteoporosis: a three-year study. J Bone Miner Res. 2013;28(3):449-54. [ Links ]
31 Saag KG, Agnusdei D, Hans D, Kohlmeier LA, Krohn KD, Leib ES, et al. 2016. Trabecular Bone Score in patients with chronic glucocorticoid therapy-induced osteoporosis treated with alendronate or teriparatide. Arthritis Rheumatol. 2016;68(9):2122-8. [ Links ]
32 Shin MS, Cho EH, Kim HY. Longitudinal change in Trabecular Bone Score during and after treatment of osteoporosis in postmenopausal Korean women. J Bone Metab. 2017;24(2):117-24. [ Links ]
33 Tsai JN, Jiang LA, Lee H, Hans D, Leder BZ. Effects of teriparatide, denosumab, or both on spine trabecular microarchitecture in DATA-Switch: a Randomized Controlled Trial. J Clin Densitom. 2017;20(4):507-12. [ Links ]
34 Watts NB, Aggers D, McCarthy EF, Savage T, Martinez S, Patterson R, et al. 2017. responses to treatment with teriparatide in patients with atypical femur fractures previously treated with bisphosphonates. J Bone Miner Res. 2017;32(5):1027-33. [ Links ]
35 Shepherd JA, Schousboe JT, Broy SB, Engelke K, Leslie WD. Executive summary of the 2015 ISCD position development conference on advanced measures from DXA and QCT: fracture prediction beyond BMD. J Clin Densitom. 2015;18:274-86. [ Links ]
36 Martineau P, Leslie WD. Trabecular Bone Score (TBS): Method and applications. Bone. 2017;104:66-72. [ Links ]
37 Dhaliwal R, Cibula D, Ghosh C, Weinstock RS, Moses AM. Bone quality assessment in type 2 diabetes mellitus. Osteoporos Int. 2014;25(7):1969-73. [ Links ]
38 Kim JH, Choi HJ, Ku EJ, Kim KM, Kim SW, Cho NH, et al. Trabecular bone score as an indicator for skeletal deterioration in diabetes. J Clin Endocrinol Metab. 2015;100(2):475-82. [ Links ]
39 Caffarelli C, Giambelluca A, Ghini V, Francolini V, Pitinca MDT, Nuti R, et al. In type-2 diabetes subjects trabecular bone score is better associated with carotid intima-media thickness than BMD. Calcif Tissue Int. 2017;101(4):404-11. [ Links ]
40 Bonaccorsi G, Fila E, Messina C, Maietti E, Ulivieri FM, Caudarella R, et al. Comparison of trabecular bone score and hip structural analysis with FRAX(R) in postmenopausal women with type 2 diabetes mellitus. Aging Clin Exp Res. 2017;29(5):951-7. [ Links ]
41 Holloway KL, De Abreu LLF, Hans D, Kotowicz MA, Sajjad MA, Hyde NK, et al. Trabecular Bone Score in men and women with impaired fasting glucose and diabetes. Calcif Tissue Int. 2018;102(1):32-40. [ Links ]
42 Iki M, Fujita Y, Kouda K, Yura A, Tachiki T, Tamaki J, et al. Hyperglycemia is associated with increased bone mineral density and decreased trabecular bone score in elderly Japanese men: The Fujiwara-kyo osteoporosis risk in men (FORMEN) study. Bone. 2017;105:18-25. [ Links ]
43 Leslie WD, Aubry-Rozier B, Lamy O, Hans D. TBS (trabecular bone score) and diabetes-related fracture risk. J Clin Endocrinol Metab. 2013;98(2):602-9. [ Links ]
44 Zhukouskaya VV, Eller-Vainicher C, Gaudio A, Privitera F, Cairoli E, Ulivieri FM, et al. The utility of lumbar spine trabecular bone score and femoral neck bone mineral density for identifying asymptomatic vertebral fractures in well-compensated type 2 diabetic patients. Osteoporos Int. 2016;27(1):49-56. [ Links ]
45 Choi YJ, Ock SY, Chung YS. Trabecular Bone Score (TBS) and TBS-adjusted fracture risk assessment tool are potential supplementary tools for the discrimination of morphometric vertebral fractures in postmenopausal women with type 2 diabetes. J Clin Densitom. 2016;19(4):507-14. [ Links ]
46 Leslie WD, Krieg MA, Hans D. Clinical factors associated with trabecular bone score. J Clin Densitom. 2013; 16(3):374 9. [ Links ]
47 Leib ES, Winzenrieth R. Bone status in glucocorticoid-treated men and women. Osteoporos Int. 2016;27(1):39-48. [ Links ]
48 Paggiosi MA, Peel NFA, Eastell R. The impact of glucocorticoid therapy on trabecular bone score in older women. Osteoporos Int. 2015;26(6):1773-80. [ Links ]
49 Chuang MH, Chuang TL, Koo M, Wang YF. Trabecular Bone Score reflects trabecular microarchitecture deterioration and fragility fracture in female adult patients receiving glucocorticoid therapy: a pre-post controlled study. Biomed Res Int. 2017;2017:4210-217. [ Links ]
50 Eller-Vainicher C, Morelli V, Ulivieri FM, Palmieri S, Zhukouskaya VV, Cairoli E, et al. Bone quality, as measured by trabecular bone score in patients with adrenal incidentalomas with and without subclinical hyper-cortisolism. J Bone Miner Res. 2012;27(10):2223-30. [ Links ]
51 Belaya ZE, Hans D, Rozhinskaya LY, Dragunova NV, Sasonova NI, Solodovnikov AG, et al. The risk factors for fractures and trabecular bone-score value in patients with endogenous Cushing's syndrome. Arch Osteoporos. 2015;10:44. [ Links ]
52 Eller-Vainicher C, Filopanti M, Palmieri S, Ulivieri FM, Morelli V, Zhukouskaya VV, et al. Bone quality, as measured by trabecular bone score, in patients with primary hyperparathyroidism. Eur J Endocrinol. 2013;169(2):155 62. [ Links ]
53 Silva BC, Boutroy S, Zhang C, McMahon DJ, Zhou B, Wang J, et al. Trabecular bone score (TBS) -a novel method to evaluate bone microarchitectural texture in patients with primary hyperparathyroidism. J Clin Endocrinol Metab. 2013;98(5):1963-70. [ Links ]
54 Diaz-Soto G, de Luis Roman D, Jauregui OI, Briongo L, Romero E, Perez-Castrillon JL. Trabecular bone score in patients with normocalcemic hyperparathyroidism. Endocr Pract. 2016;22(6):703-7. [ Links ]
55 Cipriani C, Abraham A, Silva BC, Cusano NE, Rubin MR, McMahon DJ, et al. Skeletal changes after restoration of the euparathyroid state in patients with hypoparathyroidism and primary hyperparathyroidism. Endocrine. 2017;55(2):591-8. [ Links ]
56 Rolighed L, Rejnmark L, Sikjaer T, Heickendorff L, Vestergaard P, Mosekilde L, et al. Vitamin D treatment in primary hyperparathyroidism: a randomized placebo controlled trial. J Clin Endocrinol Metab. 2014;99(3):1072-80. [ Links ]
57 Kolta S, Briot K, Fechtenbaum J, Paternotte S, Armbrecht G, Felsenberg D, et al. TBS result is not affected by lumbar spine osteoarthritis. Osteoporos Int. 2014;25(6):1759-64. [ Links ]











 text in
text in