My SciELO
Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista de Osteoporosis y Metabolismo Mineral
On-line version ISSN 2173-2345Print version ISSN 1889-836X
Rev Osteoporos Metab Miner vol.11 n.2 Madrid Apr./Jun. 2019 Epub Jan 20, 2020
https://dx.doi.org/10.4321/s1889-836x2019000200003
ORIGINALS
A novel therapeutic target for osteoarthritis: control of cellular plasticity and senescence using connexin43
1Grupo de Investigación Traslacional en Comunicación y Señalización Celular (CellCOM) - Instituto de Investigación Biomédica de A Coruña (INIBIC) - Servicio Gallego de Salud (SERGAS) - Universidad de A Coruña (UDC) - A Coruña (España)
2Tecnologías Principales de Citometría de Flujo del Instituto Conway de Investigación Biomédica y Biomolecular - Universidad de Dublín - Dublín (Irlanda)
3Departamento de Cirugía Ortopédica y Traumatología - Complejo Hospitalario Universitario de Santiago de Compostela (CHUS) - Universidad de Santiago de Compostela (USC) - Santiago de Compostela (España)
Introduction: Osteoarthritis (OA) is a degenerative musculoskeletal disease, which affects approximately the 13% of western population. Nowadays, there is no effective treatment for OA to avoid disease progression or to promote cartilage regeneration. Connexin43 (Cx43) is a transmembrane protein increased in cartilage and synovium from OA patients. Cx43 forms membrane channels that allow the exchange of molecules and ions between two adjacent cells through gap junctions (GJs), or between a cell and its environment through hemichannels. In this study we investigated the involvement of Cx43 and GJ intercellular communication in the degradation of articular cartilage in chondrocytes from patients with OA. Material and methods: Primary chondrocytes were obtained from cartilage from OA and healthy donors. Protein levels were evaluated by western-blot, immunofluorescence and flow cytometry. RNA expression was evaluated by RT-qPCR. A scrape loading/dye transfer assay was used to evaluate cell communication. Cell senescence was analysed by flow cytometry or by light microscopy using βgalactosidase assay. Results: Cx43 and GJs overactivities were correlated with the progression of OA, by promoting chronic cell dedifferentiation and senescence in vitro assays. We found that Cx43 overexpression activates factors involved in epithelial-to-mesenchymal transition, such as Twist-1. Increased levels of dedifferentiated cells, with high rates of cell proliferation, led to cell senescence via p53/p16INK4a, activating the senescence-associated secretory phenotype (SASP) and promoting the synthesis and liberation of inflammatory factors, including the interleukin-6 (IL-6). Cx43 downregulation by using small molecules, such as oleuropein, or by genetic edition with CRISPR technology, led to the chondrocyte redifferentiation and an improved phenotype, with increased synthesis of extracellular matrix proteins such as Col2A1 and downregulating the synthesis of MMPs, inflammation and senescence. Conclusions: Downregulation of Cx43 in OA chondrocytes restores regeneration by activating chondrocyte re-differentiation and decreasing cellular senescence. These results corroborate the use of Cx43 as an effective therapeutic target in order to restore cartilage regeneration and avoid OA progression.
Key words connexin43; osteoarthritis; dedifferentiation; senescence; tissue regeneration
INTRODUCTION
Osteoarthritis (OA) is a chronic disease that is characterized by a progressive degradation of the articular cartilage that covers the surface of the synovial joints, which allow the movement of the skeleton without causing pain. Chondrocytes from patients with osteoarthritis undergo changes in the phenotype associated with an increase in catabolic and inflammatory activity1,2, along with an increase in cellular senescence and senescence-associated secretory phenotype (SASP)2,3. Our research group has previously shown that chondrocytes in the articular cartilage have long cytoplasmic projections that cross the extracellular matrix (ECM)4, which form connections and gap junctions (GJs) through connexin-43 channels (Cx43)4,5. In 2013, our research group published relevant results associated with alterations in the activity of Cx43 in osteoarthritis, indicating that from the disease’s early stages there is an increase and changes in the localization of the protein in the cartilage of patients with arthrosis6. Subsequently, using animal models, we observed that the C-terminal domain of Cx43 plays a fundamental role in the structure and composition of articular cartilage7.
Cx43 is involved in tissue regeneration processes in skin, heart and other tissues8,9. Several authors have reported that osteoarthritis could be included in diseases related to alterations in tissue regeneration10,11. In fact, arthritic chondrocytes undergo cellular dedifferentiation and present higher levels of cell proliferation12,13, probably due to an attempt to repair the damage produced in the cartilage. The presence of chronically dedifferentiated chondrocytes triggers the progressive replacement of articular cartilage by fibrocartilage associated with degeneration and functional loss in the joint14-19. In this line of research, it is important to emphasize that the use of molecules that promote chondrogenesis, and therefore the redifferentiation of the chondrocyte, have a protective effect in OA20 models. These molecules are called OA-modifying drugs (DMOADs), among which is kartogenin, which has been shown to promote chondrogenesis in human mesenchymal stem cells and also improve regeneration. of cartilage in mice subjected to inflammatory and/or mechanical damage in the joint20. Other DMOADs, such as TD-198946, TAK-778 or AG-041R, have also been described as promoter molecules of chondrogenesis with therapeutic potential in repair of articular cartilage21-23. The cartilage of patients with OA presents high levels of Cx43 together with alterations in the process of tissue regeneration. Our objective was to study whether alterations in Cx43 activity and intercellular communication through UCs would be related to changes in the cellular phenotype and senescence associated with disease progression.
MATERIAL AND METHODS
Sample collection and cell culture
The cartilage samples were isolated and processed as previously described4 after the donors signed the informed consent and the approval of the Clinical Research Ethics Committee of Galicia (C.0003333, 2012/094 and 2015/029) was granted. We used the human chondrocyte cell line T/C-28a2, from healthy primary chondrocytes that were transfected with the SV40 virus particle, donated by Dr. Mary Goldring (The Hospital for Special Surgery, New York, USA). The chondrocytes were cultured in DMEM medium (Dulbecco's modified Eagle's Medium, Lonza) supplemented with 10% fetal bovine serum (FBS) and a mixture of 1% antibiotics (P/S; Penicillin 100 U/mL, Streptomycin 100 μg/mL, Gibco).
Western blot
The analysis of total or nuclear protein levels was carried out using the Western blot technique. Equivalent amounts of proteins were separated in 10% denaturing acrylamide gels and transferred to a polyvinylidene fluoride (PVDF) membrane. After blocking with skimmed milk diluted in tris buffered-saline (TBS), the membranes were incubated overnight at 4°C with anti-α-tubulin primary antibodies (Sigma-Aldrich, T9026), Cx43 (Sigma-Aldrich, C6129) Twist-1 (SCBT, sc-81417), cell proliferation nuclear antigen or PCNA (SCBT, sc-56), p53 (SCBT, sc-126), nuclear factor enhancer of the kappa light chains of activated B cells or NF-Kß ( SCBT, sc8008) or Lamin A (SCBT, sc-20680). After incubation with the primary antibody, the membranes were washed with TBS and incubated with their corresponding secondary antibodies labeled with horseradish peroxidase (HRP) for 1 hour at room temperature. Once the excess antibody was removed with TBS, the signal was visualized in a LAS-3000 development chamber (Fujifilm).
Immunofluorescence
For protein detection by immunofluorescence, cells previously fixed with 2% paraformaldehyde were incubated with 0.1 M glycine (Sigma-Aldrich) for 10 minutes. Subsequently, a permeabilization of the cell membranes was performed with Triton X-100 (Sigma-Aldrich) at 0.2% in phosphate buffered saline (PBS), for 10 minutes. Nonspecific junctions were blocked by a 30-minute incubation with 1% bovine albumin serum (BSA, Sigma-Aldrich) in PBS. Subsequently, the cells were incubated with the primary antibody for 1 hour at room temperature. After three 10-minute washes with PBS, the cells were incubated with the secondary antibody labeled with a fluorophore for 1 hour, in the dark and at room temperature. Three more 10-minute washes were carried out with PBS, followed by a staining of 4 'nuclei, 6-diamidino-2-phenylindol-DAPI- (Sigma-Aldrich). The images were made in an Olympus BX61 microscope with a DP71 camera.
Immunohistochemistry
Chondrocyte micromases were embedded in O.C.T.TM (Optimum Cutting Temperature) compound and cut into 4 µm sections, which were incubated with 3% hydrogen peroxide for 10 minutes. Sections were incubated with the primary anti-collagen type II antibody for 1 hour at room temperature. After three washes with PBS, the sections were incubated with OptiView HQ Universal Linker (Roche) for 10 minutes. Subsequently, they were incubated for 8 minutes with OptiView HRP Multimer (Roche), the excess reagent was washed and the signal was revealed in a solution of 0.1% DAB in 0.02% hydrogen peroxide.
Flow cytometry
For the measurement of protein levels by flow cytometry, the cells were fixed with 1% paraformaldehyde for 10 minutes, washed with a wash solution (PBS + 0.5% BSA + 2mM EDTA), and stained with antibodies anti-Cx43-APC (R & D Systems, FAB7737A), endoglin or CD105-PE (Immunostep, 105PE-100T) or CD166 antigen (ALCAM) or CD166-APC (Immunostep, 1399990314). The analysis was carried out on a FACSCaliburTM cytometer.
Cell transfection
The T/C-28a2 cell line was transfected by electroporation with the Amaxa® Cell Line Kit Nucleofector® kit V (Lonza) in a Cell Line NucleofectorTM (Lonza). One million cells were electroporated with 3 μg of plasmid pIRESpuro2 (Clontech) containing the sequence of the human Cx43 gene, donated by Dr. Arantxa Tabernero (INCYL, University of Salamanca, Spain). At 24 hours the medium was changed by means of P/S and antibiotic for the selection of the chondrocytes containing the plasmid.
On the other hand, the electroporation of the T/C28a2 line was also carried out with a CRISPR vector (modified from Addgene #48138) with the enzyme Cas9 VP12 (derived from Addgene #72247) bound to a GFP marker (green protein). fluorescent), with a guide that targets 20 nucleotides of the Cx43 gene. This vector has been donated by Dr. Trond Aasen (Vall d'Hebron Research Institute, Autonomous University of Barcelona, Spain). Electroporated and positive cells for GFP were seeded in a 96-well plate and expanded as clones.
Gene expression
Gene expression levels were carried out by extracting mRNA with TRIzol (Invitrogen), retrotranscription with SuperScript® VILO ™ kit (Invitrogen) and quantification by quantitative real-time PCR in a LightCycler® 480 (Roche). Primers were used for:
hypoxanthine phosphoribosyltransferase-1 -HPRT-1(5'-TTGAGTTTGGAAACATCTGGAG-3 '; 5'-GCCCAAAGG-GAACTGATAGTC-3'), - GJA1 (5'-ACATGGGTGACTG-GAGCGCC-3 '; 5'-ATGATCTGCAGGACCCAGAA-3'),
interleukin-1. -IL-1β.(5'-CGAATCTCCGACCACCAC-TAC-3 '; 5' - TCCATGGCCACAACAACTGA-3 '),
interleukin-6 -IL-6-(5 '-TGTAGCCGCCCCACACA-3'; 5 '-GGATGTACCGAATTTGTTTGTA-3'),
prostaglandin-endoperoxide synthase-2 -PTGS2- (5'-CTTCACGCATCAGTTTTTCAAG -3 '; 5'-TCACCGTAAATAT-GATTTAAGTCCAC-3'),
metalloprotease 3 -MMP-3-(5'-CCCTGGGTCTCTTT CACTCA-3 '; 5'-GCTGACAGCATCAAAGGACA-3'),
cyclin-dependent kinase inhibitor-2 -CDKN2-(5'-GAGCAGAACGATAGGGCTTG-3 '; 5'-CAT GTGCCCTCT CCTCCTAA-3').
CUs Activity
Cell communication through communicating junctions was evaluated using a Scrape Loading/Dye Transfer (SL/DT) test. For this, a cut is made on confluent cells with a scalpel and the tip of a needle in Lucifer Yellow fluorescent compound (LY, Cell Projects Ltd© Kent, UK), incubating at 37°C for 5 minutes. The damaged cells that manage to repair the membrane take the fluorescent compound from the medium. The transfer of LY from the cut line was evaluated in an inverted fluorescence microscope (Nikon Eclipse Ti) and the ratio between undamaged cells positive for LY was calculated between the number of cells taking the compound through a damage in the membrane.
Senescence
Cellular senescence was evaluated according to β-galactosidase activity with a commercial kit with X-gal as a substrate (Senescence Cells Histochemical Kit, Sigma-Aldrich) and also by flow cytometry with the substrate di-β-galactopyranoside, which results in green fluorescence when hydrolyzed (Invitrogen). In the case of X-gal, the cells with β-galactosidase activity will be stained greenish blue, so that they can be analyzed under a visible light microscope. On the other hand, the hydrolysis of the di-β-galactopyranoside substrate was detected on a FACSCalibur™ cytometer, and the mean fluorescence was normalized to the untreated cell levels.
RESULTS
Cx43 activates the catabolic activity in chondrocytes of patients with OA
Concurring with what was observed in tissue6, articular chondrocytes in primary culture from donors with OA (OAc) had significantly higher Cx43 levels than those isolated from healthy donors (N) detected by flow cytometry (Figure 1A). The high levels of Cx43 were correlated with higher levels of intercellular communication through UCs, quantified by an SL/DT transfer assay of LY (Figure 1B). In order to study the effect on the cellular phenotype of high levels of Cx43 and intercellular communication through UCs, a healthy donor chondrocyte cell line, T/C-28a2, was used as a study model. Cx43 was overexpressed using a vector with the human Cx43 gene under the CMV24 promoter (Figure 1C). The increase in Cx43 in the human chondrocyte cell line T/C-28a2 was correlated with an increase in the activity of the UCs detected by the SL/DT assay (Figure 1D). The gene expression assay by RT-PCR showed a significant increase in the gene expression of interleukin 1-β (IL-1β), cyclooxygenase-2 (COX-2) and metalloprotease-3 (MMP-3) when the Cx43 was overexpressed in the healthy chondrocyte line (T/C - Cx43) (Figure 1E).
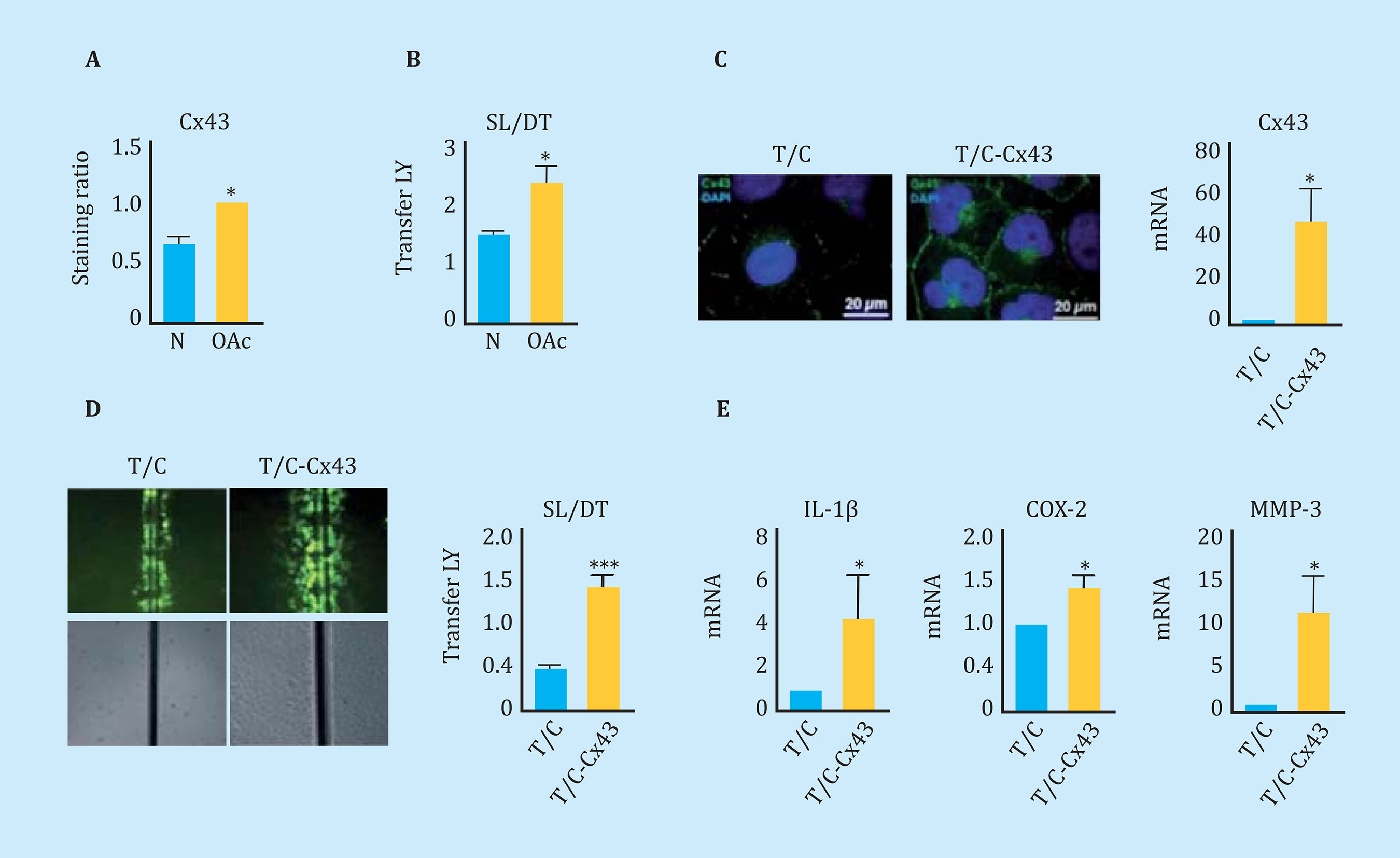
Figure 1 (A) Cx43 levels analyzed by flow cytometry comparing healthy (N) and osteoarthritic (OAc) human chondrocytes in monolayer culture. n=3, mean ± standard error of the mean (EEM); ***p<0.0001; Student t test. (B) Quantification of the Scrape Loading/Dye Transfer (SL/DT) cellular communication assay comparing chondrocytes from healthy (N) and osteoarthritic (OAc) donors. n=8, mean ± SEM; **p<0.01; Mann-Whitney test. (C) On the left, immunofluorescence for Cx43 (green) in chondrocytes T/C-28a2 (T/C) and the same line transfected with a plasmid to overexpress Cx43 (T/C-Cx43). The nuclei have been stained with DAPI (blue). On the right, gene expression levels of Cx43 in these two chondrocyte lines. n=5, mean ± SEM; *p<0.05; Mann-Whitney test. (D) Quantification of the SL/DT cellular communication assay, comparing the T/28a2 (T/C) line and transfected with a plasmid to overexpress the Cx43 (T/C-Cx43). n=10, mean ± SEM; ***p<0.0001; Mann-Whitney test. (E) Levels of gene expression of IL-1β, COX-2 and MMP-3 in the T/C-28a2 line that over-expresses Cx43 (T/C-Cx43) compared to the line transfected with a control plasmid (T/C). n=4, mean ± SEM; *p<0.05; Mann-Whitney test
Activation of cell dedifferentiation in OA
Using flow cytometric assays, we studied the levels of cell de-differentiation markers in chondrocytes from patients with osteoarthritis and chondrocytes isolated from healthy donors, in order to confirm the presence of immature chondrocytes in cartilage samples from patients with OA. By flow cytometry, higher levels of the CD166 "Stem" marker were detected in OAc in primary culture compared to healthy chondrocytes (Figure 2A). Consistent with these results, the increase of Cx43 in healthy chondrocytes (cell line) using an expression vector (T/C-Cx43 or T/C-28a2 line transfected with a plasmid to overexpress Cx43) triggered a significant increase in the levels of the two "stem-like" markers CD166 and CD105, with respect to the control cells with low levels of Cx43 (T/C-28a2) (Figure 2B).
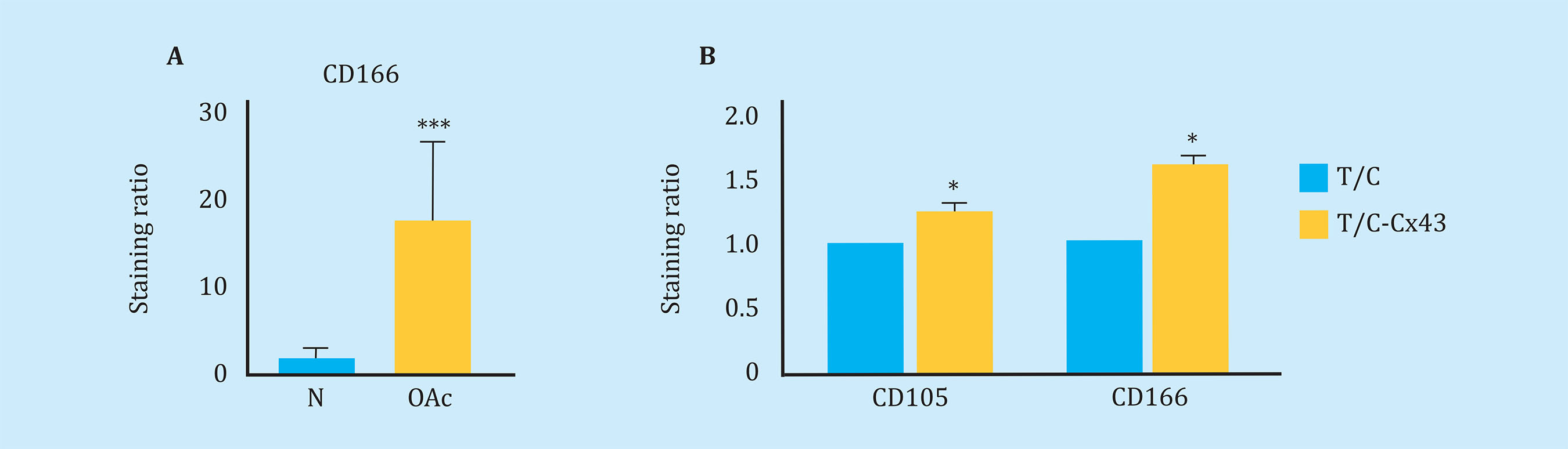
Figure 2 (A) Measurement of CD166 dedifferentiation marker by flow cytometry in arthritic chondrocytes (OAc) and chondrocytes from healthy donors (n=9, mean ± SEM, ***p<0.0001, Mann-Whitney test). (B) Levels of markers CD105 (n=5) and CD166 (n=7) measured by flow cytometry in the T/C-28a2 cell line that over-expresses Cx43 (T/C-Cx43) compared to the same line transfected with a control plasmid (T/C). Mean ± EEM; *p<0.05; Mann-Whitney test
The decrease in the activity of the Cx43 and the UCs activates cellular re-differentiation in OA
To reduce Cx43 activity in OAc, the effect of different molecules on the levels and activity of Cx43 was studied. In this study, we observed that the polyphenol oleuropein decreases the levels of Cx43 in OAc (Figure oleuropein decreases the levels of Cx43 in OAc (Figure 3A). The decrease in Cx43 levels improved the OA chondrocyte phenotype detected by an increase in the main marker of articular chondrocytes, collagen II (Figure 3B). The treatment of OAc with a concentration of 10 μM of oleuropein for 7 days significantly decreased levels of CD105 and CD166 dedifferentiation markers (Figure 3C), as well as the gene expression of IL-1β, IL-6, COX-2 and MMP-3 detected by flow cytometry and analysis of gene expression respectively (Figure 3D). The effect of Cx43 on cellular plasticity in OAc was confirmed in 3D culture. Modulation of Cx43 levels in the presence of 10 μM oleuropein in micromasses and in chondrogenic medium improved the structure of the extracellular matrix, detecting a significant increase in collagen II deposits and proteoglycans in the 3D structure matrix (Figure 4).
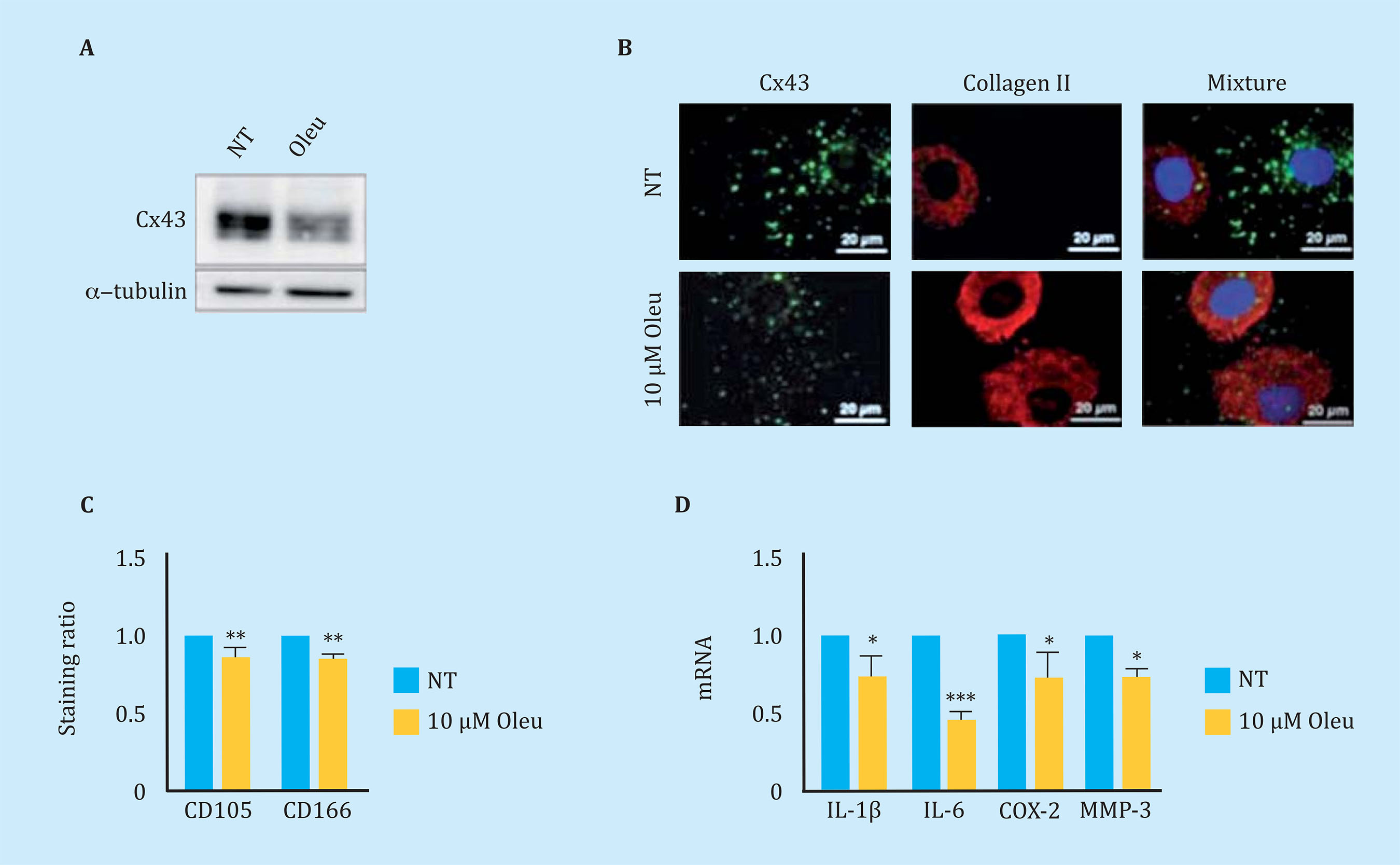
Figure 3 (A) Western blot to detect Cx43 in arthritic chondrocytes (OAc) in primary culture untreated (NT) or treated with 10 μM oleuropein (Oleu) for 2 hours. (B) Co-immunofluorescence of Cx43 (green) and collagen type II (red) of OAc treated with 10 μM oleuropein for 2 h. The cell nuclei appear in blue due to DAPI staining. The white arrows indicate Cx43 located in the cell membrane. (C) Levels of CD105 and CD166 markers measured by flow cytometry in OAc treated with 10 μM oleuropein for 7 days (n=5, mean ± SEM, **p<0.01, Mann-Whitney test). (D) Gene expression levels of IL-1β, IL-6, COX-2 and MMP-3 in OAc treated with 10 μM oleuropein for 2 hours (n=3-7, mean ± SEM; *p<0.05, ***p<0.0001, Mann-Whitney test)
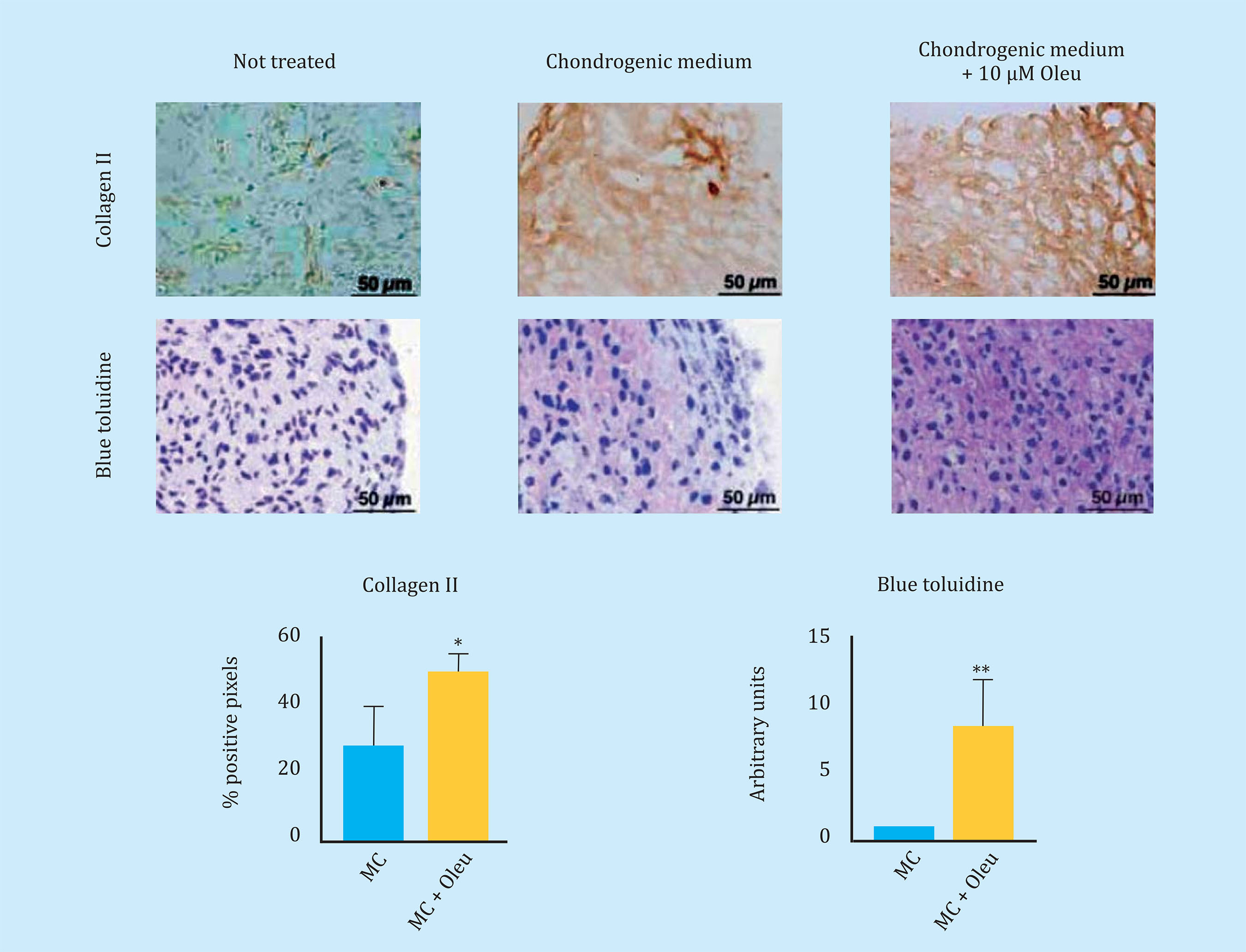
Figure 4 Sections of three-dimensional culture of arthritic chondrocytes (OAc) cultured in chondrogenic medium (MC) with/without 10 μM oleuropein for 30 days. In the upper panel, immunohistochemistry of a micromass for type II collagen (n=4-6, mean ± SEM, *p<0.05, Student's t-test). Below, staining of toluidine blue to detect proteoglycans, which produce a blue to pink-violet color shift (n=6, mean ± SEM, **p<0.01, Mann-Whitney test)
Cx43 activates TEM and cellular senescence in OAc
The overexpression of Cx43 in the line of chondrocytes T/Cx28a2, was correlated with an increase in the nucleus of PCNA, protein used as a marker of cell proliferation, and with activation of the transcription factor related to TEM, Twist-1, detected by translocation and increased levels of the transcription factor at the nuclear level (Figure 5A). The transfected chondrocytes to overexpress the Cx43 also showed higher nuclear levels of NF-kß, one of the most important transcription factors in the regulation of synthesis of the SASP compo- nent (Figure 5A). Elevated levels of Cx43 correlated with elevated levels of factors involved in p53 cellular senescence (Figure 5B) and p16 (Figure 5C). OAc treatment with 10 μM oleuropein reduced Cx43 levels (Figure 3A) and cellular senescence detected by β-galactosidase activity by light microscopy and flow cytometry (Figure 5D). In order to confirm the effect of the Cx43 decrease in TEM and cellular senescence, the T/Cx28a2 line was transfected with a CRISPR/Cas9 plasmid, obtaining heterozygous cells for the Cx43 gene (Figure 6A). Reduced levels of Cx43 on the T/Cx28a2 line correlated with a significant decrease in the "stemlike" markers CD166 and CD105 (Figure 6B). The decrease in Cx43 levels in these cells, triggered a decrease in the levels of transcription factors Twist-1 (TEM) and NF-kβ (SASP) at the nuclear level (Figure 6C), decreasing the levels of cellular senescence , detected by β-galactosidase activity and flow cytometry (Figure 6D). T/Cx28a2 chondrocytes with low levels of Cx43 (CRISPR-Cx43) showed significantly lower levels of synthesis of the pro-inflammatory mediators IL-1β and IL-6, and of protease MMP-3, with respect to the line T/C-28a2 without transfecting.
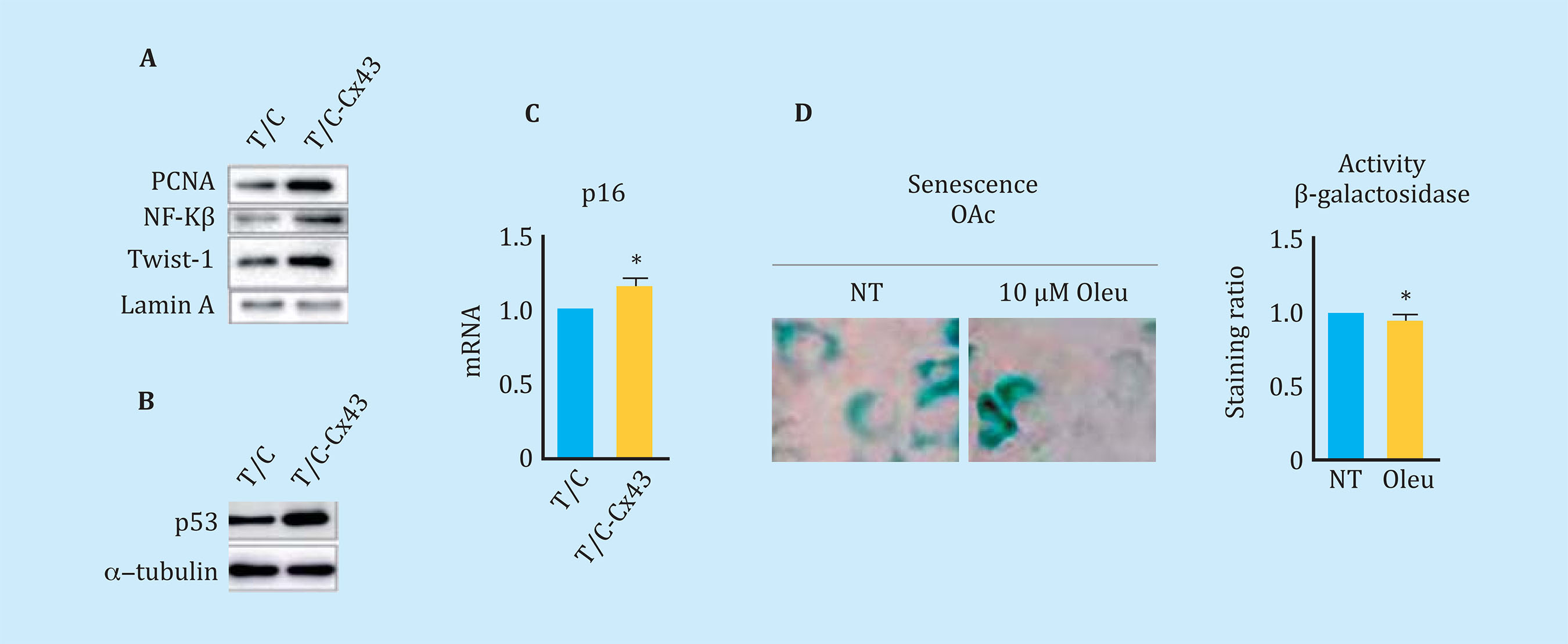
Figure 5 (A) Western blot comparing levels of PCNA, NF-Kβ, and nuclear Twist-1 in chondrocytes that over-express Cx43 (T/C-Cx43) with respect to the same chondrocytes transfected with a control plasmid (T/C). (B) Western blot comparing total p53 levels between chondrocytes overexpressing Cx43 (T/C-Cx43) and control chondrocytes (T/C). (C) Gene expression of p16 of chondrocytes overexpressing Cx43 with respect to control cells (n=4, mean ± SEM, *p<0.05, Mann-Whitney test). (D) Above, β-galactosidase staining associated with senescence measured by X-gal rupture in arthritic chondrocytes (OAc) treated with 10 μM oleuropein for 7 days. Below, quantification by flow cytometry of β-galactosidase levels after the same treatment (n=5, mean ± SEM, *p<0.05, Mann-Whitney test)
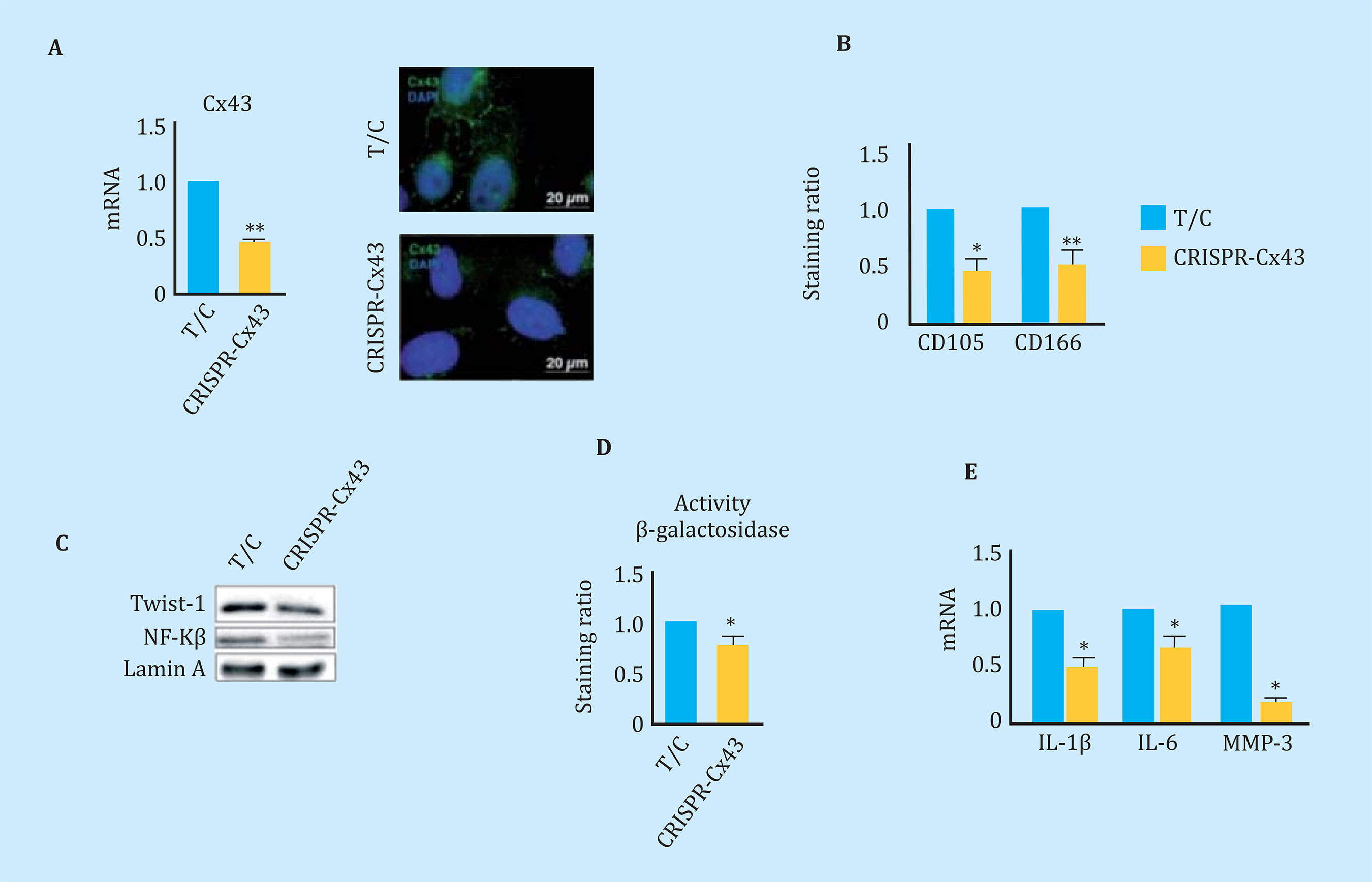
Figure 6 (A) On the left, gene expression levels of Cx43 in chondrocytes T/C-28a2 (T/C) and the same line with only one copy of Cx43 (CRISPR-Cx43). n=4, mean ± SEM; *p<0.05; Mann-Whitney test. On the right, immunofluorescence for Cx43 (green) in chondrocytes T/C-28a2 (T/C) and the same line transfected with only one copy of Cx43 (CRISPR-Cx43). The nuclei have been stained with DAPI (blue). (B) Levels of the CD105 and CD166 markers measured by flow cytometry in the T/C-28a2 cell line with a single copy of Cx43 (CRISPR-Cx43) compared to the same line without transfection (T/C). n=7, mean ± SEM; *p<0.05, **p<0.01; Mann-Whitney test (C) Western blot to detect Twist-1, NFkβ and N-Cadherin comparing a nuclear extract of the T/C-28a2 (T/C) line and from the same line with low amount of Cx43 (CRISPR-Cx43). Lamina protein A has been used as a load control. (D) Quantification by flow cytometry of β-galactosidase levels in the T/C-28a2 line (T/C) and the same line with only one copy of Cx43 (CRISPR-Cx43). n=4, mean ± SEM; *p<0.05; Mann-Whitney test. (E) Gene expression levels of IL-1β, IL-6 and MMP-3 measured in the same cells. n=4, mean ± SEM; *p<0.05; Mann-Whitney test)
DISCUSSION
During osteoarthritis, the chondrocytes have increased levels of the transmembrane protein Cx436 and their phenotype is altered preventing them from participating in tissue regeneration and carrying out their function, triggering progressive tissue degeneration. The dedifferentiation related to epithelial-mesenchymal transition phenomena (TEM) is a cellular process that participates in the regeneration of tissues by allowing the cells to dedifferentiate into a more immature state to activate processes, including cell proliferation and migration, with the objective of replacing the damaged cells and remodeling the extracellular matrix25,26. However, when this dedifferentiation occurs chronically it can cause the development of fibrosis in the context of tissue regeneration27,28. In this study we have described that the levels of Cx43 and intercellular communication through UCs in osteoarthritis correlate positively with the cell de-differentiation markers CD105 and CD166. In addition, we have verified that this state can be partially reversed by the use of molecules that decrease the levels of Cx43, improving the phenotype of arthritic chondrocytes and
MEC in in vitro tests. The decrease in Cx43 gave rise to cellular re-differentiation and, therefore, to a lower expression of pro-inflammatory cytokines and degrading enzymes of the articular cartilage matrix. Our results also show that high levels of Cx43 in chondrocytes are related to an increase in senescence associated with a higher expression of p16INK4a and high levels of p53. Recent studies highlight the importance of senescence in osteoarthritis29-33. In fact, Jeon et al. published an article in Nature Medicine where they showed senescence as a new therapeutic target to treat osteoarthritis and promote the regeneration of cartilage33,34. In this study we demonstrate for the first time the relationship between the over activity of Cx43 in human chondrocytes and the activation of dedifferentiation and cellular senescence that lead to alterations in the regeneration process and favor the progress of the disease. From these results, therapies aimed at decreasing Cx43 levels in osteoarthritis arise as an interesting therapeutic approach for osteoarthritis.
In conclusion, these findings suggest that the increase in Cx43 activity reached from very early stages of OA6 could contribute to the degeneration of articular cartilage and joint by activating cellular dedifferentiation via TEM and cellular senescence, contributing to the synthesis of enzymes that degrade the release of cytokines that contribute to the degenerative process in the joint. These results demonstrate that Cx43 and UCs act as a regulator of dedifferentiation/re-differentiation and senescence in chondrocytes, probably activating proteins related to TEM, such as Twist-1, and pro-inflammatory cytokines such as IL-1β. The decrease in Cx43 levels in OAc promotes its re-differentiation, decreasing the expression of inflammatory mediators and senescence, and in turn is accompanied by a greater deposition of Col2A1 and proteoglycans in the extracellular matrix. The use of molecules such as oleuropein and the design of studies to decrease the activity of Cx43 in vivo is probably a first step in the development of innovative therapeutic strategies for the effective treatment of osteoarthritis from early stages of the disease by restoring tissue regeneration.
Bibliografía
1 Kapoor M, Martel-Pelletier J, Lajeu-nesse D, Pelletier JP, Fahmi H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat Rev Rheumatol. 2011;7(1):33-42. [ Links ]
2 Loeser RF, Collins JA, Diekman BO. Ageing and the pathogenesis of osteoarthritis. Nat Rev Rheumatol. 2016; 12(7):412-20. [ Links ]
3 Philipot D, Guerit D, Platano D, Chu-chana P, Olivotto E, Espinoza F, et al. p16INK4a and its regulator miR-24 link senescence and chondrocyte terminal differentiation-associated matrix remodeling in osteoarthritis. Arthritis Res Ther. 2014;16(1):R58. [ Links ]
4 Mayan MD, Gago-Fuentes R, Carpin-tero-Fernandez P, Fernandez-Puente P, Filgueira-Fernandez P, Goyanes N, et al. Articular chondrocyte network mediated by gap junctions: role in metabolic cartilage homeostasis. Ann Rheum Dis. 2013. [ Links ]
5 Chi SS, Rattner JB, Matyas JR. Communication between paired chondrocytes in the superficial zone of articular cartilage. J Anat. 2004;205(5):363-70. [ Links ]
6 Mayan MD, Carpintero-Fernandez P, Gago-Fuentes R, Martinez-de-Ilarduya O, Wang HZ, Valiunas V, et al. Human articular chondrocytes express multiple gap junction proteins: differential expression of connexins in normal and osteoarthritic cartilage. Am J Pathol. 2013;182(4):1337-46. [ Links ]
7 Gago-Fuentes R, Bechberger JF, Varela-Eirin M, Varela-Vazquez A, Acea B, Fonseca E, et al. The C-terminal domain of connexin43 modulates cartilage structure via chondrocyte phenotypic changes. Oncotarget. 2016;7(45). [ Links ]
8 Mendoza-Naranjo A, Cormie P, Serrano AE, Wang CM, Thrasivoulou C, Sutcliffe JE, et al. Overexpression of the gap junction protein Cx43 as found in diabetic foot ulcers can retard fibroblast migration. Cell Biol Int. 2012;36[7):661-7. [ Links ]
9 Brandner JM, Houdek P, Husing B, Kaiser C, Moll I. Connexins 26, 30, and 43: differences among spontaneous, chronic, and accelerated human wound healing. J Invest Dermatol. 2004;122[5): 1310-20. [ Links ]
10 Rai MF, Schmidt EJ, McAlinden A, Che-verud JM, Sandell LJ. Molecular insight into the association between cartilage regeneration and ear wound healing in genetic mouse models: targeting new genes in regeneration. G3. 2013;3 (11):1881-91. [ Links ]
11 Rai MF, Sandell LJ. Regeneration of articular cartilage in healer and non-healer mice. Matrix Biol. 2014;39:50-5. [ Links ]
12 Grogan SP, Miyaki S, Asahara H, D'-Lima DD, Lotz MK. Mesenchymal progenitor cell markers in human articular cartilage: normal distribution and changes in osteoarthritis. Arthritis Res Ther. 2009;11(3):R85. [ Links ]
13 Kim AC, Spector M. Distribution of chondrocytes containing alpha-smooth muscle actin in human articular cartilage. J Orthop Res. 2000;18[5):749-55. [ Links ]
14 Bae DK, Yoon KH, Song SJ. Cartilage healing after microfracture in osteo-arthritic knees. Arthroscopy. 2006;22 (4):367-74. [ Links ]
15 Bank RA, Soudry M, Maroudas A, Mizrahi J, TeKoppele JM. The increased swelling and instantaneous deformation of osteoarthritic cartilage is highly correlated with collagen degradation. Arthritis Rheum. 2000;43[10):2202-10. [ Links ]
16 Barley RD, Adesida AB, Bagnall KM, Jomha NM. Immunohistochemical characterization of reparative tissue present in human osteoarthritic tissue. Virchows Archiv. 2010;456(5):561-9. [ Links ]
17 Heinegard D, Saxne T. The role of the cartilage matrix in osteoarthritis. Nat Rev Rheumatol. 2011;7(1):50-6. [ Links ]
18 Garciadiego-Cazares D, Aguirre-San-chez HI, Abarca-Buis RF, Kouri JB, Velas-quillo C, Ibarra C. Regulation of alpha5 and alphaV Integrin Expression by GDF-5 and BMP-7 in Chondrocyte Differentiation and Osteoarthritis. PLoS One. 2015;10(5):e0127166. [ Links ]
19 Grenier S, Bhargava MM, Torzilli PA. An in vitro model for the pathological degradation of articular cartilage in osteoarthritis. J Biomech. 2014;47(3):645-52. [ Links ]
20 Johnson K, Zhu S, Tremblay MS, Payette JN, Wang J, Bouchez LC, et al. A stem cell-based approach to cartilage repair. Science. 2012;336[6082):717-21. [ Links ]
21 Yano F, Hojo H, Ohba S, Fukai A, Hosaka Y, Ikeda T, et al. A novel disease-modifying osteoarthritis drug candidate targeting Runx1. Ann Rheum Dis. 2013; 72(5):748-53. [ Links ]
22 Kitamura H, Okazaki M. AG-041R, a novel indoline-2-one derivative, stimulates chondrogenesis in a bipotent chondroprogenitor cell line CL-1. Osteoarthritis Cartilage. 2005; 13(4):287-96. [ Links ]
23 Bellesini LS, Beloti MM, Crippa GE, Bombonato-Prado KF, Junta CM, Marques MM, et al. The effect of TAK-778 on gene expression of osteoblastic cells is mediated through estrogen receptor. Exp Biol Med (Maywood). 2009;234(2):190-9. [ Links ]
24 Gangoso E, Thirant C, Chneiweiss H, Medina JM, Tabernero A. A cell-penetrating peptide based on the interaction between c-Src and connexin43 reverses glioma stem cell phenotype. Cell Death Dis. 2014;5:e1023. [ Links ]
25 Cheng F, Shen Y, Mohanasundaram P, Lindstrom M, Ivaska J, Ny T, et al. Vi-mentin coordinates fibroblast proliferation and keratinocyte differentiation in wound healing via TGF-beta-Slug signaling. Proc Natl Acad Sci U S A. 2016;113(30):E4320-7. [ Links ]
26 Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature. 2008;453(7193): 314-21. [ Links ]
27 Li M, Luan F, Zhao Y, Hao H, Zhou Y, Han W, et al. Epithelial-mesenchymal transition: An emerging target in tissue fibrosis. Exp Biol Med (Maywood). 2016;241(1):1-13. [ Links ]
28 Ye X, Weinberg RA. Epithelial-Mesenchymal Plasticity: A Central Regulator of Cancer Progression. Trends Cell Biol. 2015;25(11):675-86. [ Links ]
29 Price JS, Waters JG, Darrah C, Pennington C, Edwards DR, Donell ST, et al. The role of chondrocyte senescence in osteoarthritis. Aging Cell. 2002;1(1):57-65. [ Links ]
30 Zhou HW, Lou SQ, Zhang K. Recovery of function in osteoarthritic chondrocytes induced by p16INK4a-spe-cific siRNA in vitro. Rheumatology (Oxford). 2004;43(5):555-68. [ Links ]
31 Gao SG, Zeng C, Li LJ, Luo W, Zhang FJ, Tian J, et al. Correlation between senescence-associated beta-galactosi-dase expression in articular cartilage and disease severity of patients with knee osteoarthritis. Int J Rheum Dis. 2016;19(3):226-32. [ Links ]
32 Xu M, Bradley EW, Weivoda MM, Hwang SM, Pirtskhalava T, Decklever T, et al. Transplanted Senescent Cells Induce an Osteoarthritis-Like Condition in Mice. J Gerontol A S Biol Sci Med Sci. 2017;72(6):780-5. [ Links ]
33 Jeon OH, Kim C, Laberge RM, Demaria M, Rathod S, Vasserot AP, et al. Local clearance of senescent cells attenuates the development of post-traumatic osteoarthritis and creates a pro-regenerative environment. Nat Med. 2017; 23(6):775-81. [ Links ]
34 Toh WS, Brittberg M, Farr J, Foldager CB, Gomoll AH, Hui JH, et al. Cellular senescence in aging and osteoarthritis. Acta Orthop. 2016;87(sup363):6-14. [ Links ]
Received: October 06, 2018; Accepted: February 25, 2019











 text in
text in 


