My SciELO
Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista de Osteoporosis y Metabolismo Mineral
On-line version ISSN 2173-2345Print version ISSN 1889-836X
Rev Osteoporos Metab Miner vol.12 n.2 Madrid Apr./Jun. 2020 Epub Oct 05, 2020
https://dx.doi.org/10.4321/s1889-836x2020000200006
REVIEW
Postoperative thyroid hypocalcemia diagnosis and management protocol
1Endocrinology and Nutrition Service. Infanta Leonor University Hospital. Madrid (Spain)
2Endocrinology and Nutrition Service. San Cecilio University Hospital. Granada (Spain)
3Endocrinology and Nutrition Service. Ruber Juan Bravo Hospital. Madrid (Spain)
4Endocrinology and Nutrition Service. Rafael Méndez General University Hospital. Lorca (Spain)
5Endocrinology and Nutrition Service. Italian Hospital of Buenos Aires. Buenos Aires (Argentina)
6aGeneral Surgery Service. Ramón y Cajal University Hospital. Madrid (Spain)
6bGeneral Surgery Service. Ruber Juan Bravo Hospital. Madrid (Spain)
Objetive:
Transient hypocalcaemia due to hypoparathyroidism is the most frequent complication of cervical surgery (thyroid and parathyroid) and also of reoperations. If mild, hypocalcaemia attributed to hypoparathyroidism is associated with few symptoms or with severe symptoms such as seizures, heart failure, or laryngospasm, in severe cases. Both transient and permanent hypoparathyroidism can have important repercussions on the health of patients. Establishing appropriate protocols are required to prevent, assess and treat these conditions.
Material and methods:
A systematic bibliographic search was carried out in Pubmed.gov of available evidence from articles in English and Spanish with inclusion dates until May 2019. Recommendations were made based on the GRADE system (Grading of Recommendations, Assessment, Development and Evaluation).
Results and conclusions:
We propose a consensus for patient management of those who are going to undergo thyroid or parathyroid surgery, with different sections for the different stages of the process. This is intended to help clinical decisionmaking, assist in the discharge process and make referrals to outpatient consultations, thus optimizing resources.
Key words: hypoparathyroidism; hypocalcemia; thyroidectomy
Introduction
Transient hypocalcaemia due to hypoparathyroidism is the most common complication of cervical surgery (thyroid and parathyroid) and also of reoperations. The deficiency of parathyroid hormone (PTH) secretion causes postoperative hypocalcemia due to an inhibition of bone resorption, a decrease in the synthesis of 1-25-dihydroxy vitamin D by the kidney and reduced intestinal calcium absorption. Some associated comorbidities, such as malabsorption, gastric bypass, and bisphospho-nate therapy, may promote parathyroid failure. When PTH secretion is insufficient, hypocalcemia develops. Hypocalcaemia due to hypoparathyroidism is associated with few symptoms, if the hypocalcaemia is mild. In severe cases, symptoms include seizures, heart failure, or laryngospasm. In addition to the magnitude of hypocalcemia, the speed of establishment determines its clinical expression1.
The removal or inadvertent damage of the parathyroids or the alteration of their blood supply are the responsible causes. Both transient and permanent hypoparathyroidism can have important repercussions on patients' health and establishing appropriate protocols for their prevention, evaluation and treatment are needed2.
The frequency with which this complication appears is difficult to establish and varies according to the parameters analyzed. These parameters include the definition of hypocalcaemia, its clinical expression and the concept of transient and permanent hypoparathyroidism. A recent meta-analysis of observational studies carried out in the United Kingdom found an incidence after thyroidectomy of 27% (19-38%) for transient hypoparathyroidism, and 1% (0-3%) for permanent hypoparathyroidism3.
It is important to establish the role of the endocrinologist in the preoperative identification of patients at risk, coordinate management with the surgeon in the immediate postoperative period, and follow-up patients with prolonged hypoparathyroidism.
The aim of our proposal is to develop a protocol for the management of the patient who is going to undergo thyroid or parathyroid surgery, with various sections for the different stages of the process. This helps clinical decision-making and registration process and referral to external consultations, thus optimizing resources.
Clinical definitions
Biochemical hypoparathyroidism: biochemical hypocalcemia accompanied by PTH below the lower limit of the laboratory1.
Clinical hypoparathyroidism: biochemical hypoparathyroidism accompanied by signs or symptoms of hypocalcaemia.
Parathyroid failure or relative hypoparathyroidism: signs or symptoms of hypoparathyroidism that require medical treatment, despite normal levels1.
Transient hypoparathyroidism: hypoparathyroidism that recovers in less than 12 months.
Permanent hypoparathyroidism: hypoparathyroidism in need of treatment that lasts over 12 months.
Severe hypocalcaemia: one that presents with symptoms of carpopedal spasm, tetany, seizures, lengthening of the QT interval or hypocalcaemia that, being asymptomatic, presents acutely with corrected calcium levels less than or equal to 7.5 mg/dl, which It could lead to serious complications if left untreated.
Because, in a large part of cases, postoperative hypocal-caemia resolves in the first month after surgery, some authors choose to wait until the 4-6th week to establish the diagnosis of hypoparathyroidism, considering prolonged hypoparathyroidism if there are low PTH levels. or the patient needs treatment from one month after surgery, and permanent when this situation continues beyond one year2.
Pathophysiology
There a several mechanisms involved in postsurgical hypocalcemia. The most frequent is direct damage to the glands: either due to injury to the vascularization system, mechanical damage, or partial or complete excision of the glands inadvertently or voluntarily. The parathyroid vascularization is complex and its variants make it difficult to carry out surgery. Usually, the inferior thyroid artery is the dominant vessel, supplying both the inferior and superior parathyroids, which also tend to receive a supply from the superior thyroid artery. However, there are individuals with superior thyroid artery dominance or variants in which thyroid thymic anastomoses provide an important component in irrigation1. Thus, the surgeon's experience and ability to identify the glands and their vessels are essential in avoiding postoperative complications.
As for the causes of hypocalcemia in the postoperative period, the hungry bone syndrome deserves special mention from the pathophysiologic point of view. This syndrome is classically described in hyperparathyroid patients with significant bone involvement, in which a sudden decrease in PTH levels occurs after parathyroid surgery, leading to sustained hypocalcemia with hypophosphoremia, which may further increase if the remaining parathyroid tissue functions normally. After being chronically hypercalcemic, he is temporarily stunned4. Although a classic hungry bone syndrome would not go unnoticed, mild forms of the syndrome are possibly underdiagnosed, so it must be kept in mind at all stages of the surgical process in hyperparathyroid patients, as well as in patients with hyperthyroidism that are going to undergo thyroidectomy and present hypermetabolic bone, either through bone mineral density (BMD) or through bone remodeling markers, such as alkaline phosphatase (AF).
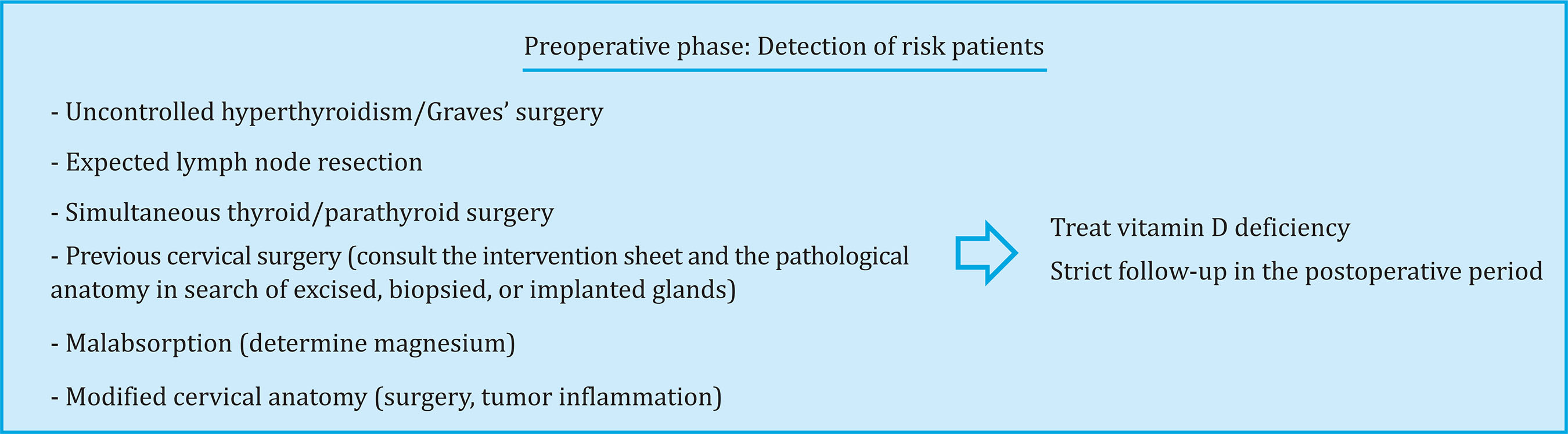
Preoperative assessment
In the patients' preoperative evaluation, we must identify those who are at increased risk of post-surgical
hypocalcemia using clinical and biochemical data (Figure 1).
As for the diseases to intervene, patients with hyperthyroidism, with tumors in which lymph node resection is also expected, or patients with simultaneous thyroid and parathyroid surgery, are at higher risk of hypocalcaemia. Likewise, patients with anatomy modified by previous cervical surgery or radiation are at higher risk.
The state of vitamin D should be assessed, since several studies have related its deficit with transient hypocalcemia3-5 7. Similarly, it is important to detect patients with malabsorptive problems and request a magnesium determination prior to the intervention.
Once the risk patients have been identified, we suggest treating vitamin D deficiency in patients who are going to undergo thyroidectomy. In the case of parathyroid surgery, although not all studies identify vitamin D deficiency as a key element in the development of postsurgical hypocalcemia8, given that several studies have shown that correction of vitamin D deficiency does not significantly increase calcaemia9,10, we suggest, if possible, to treat the deficit at least in patients with higher AF levels or bone involvement.
Recommendations:
Immediate postoperative period
Time after surgery to request initial analysis with PTH Various groups have studied the usefulness of measuring rapid or intraoperative PTH (PTHiop) and intact PTH (PTHi) in the early postoperative period, which ranges from 10 minutes to 24 hours after thyroidectomy. Depending on its levels, the short half-life of PTH (3-5 minutes) allows decision-making in the postoperative period. PTHiop is determined from blood samples drawn during or shortly after surgery. In many hospitals it provides quick results, while routine determination of intact PTH may not be fast enough to make therapeutic postop decisions11.
PTHiop levels lower than 7-17.9 pg/ml have been shown to be predictors of hypocalcemia12-14, as well as postsurgical decreases in PTH greater than 62.5-80%12,14,15. Low levels of PTHi, generally <10-15 pg/ml, in the first 24 hours postoperatively, have shown high sensitivity and specificity to predict hypocalcemia development16-20. The late decrease in iPTH, equal to or greater than 80%, has demonstrated its utility in selecting patients who are candidates for early hospital discharge21. However, the utility of early PTHi levels in predicting permanent hypoparathyroidism is the subject of controversy22.
The available evidence and the variability of the PTH measurement techniques do not allow us to clearly suggest or recommend the timing of the sample extraction or the cut-off points for deciding early hospital discharge or initiation of treatment for hypocalcaemia.
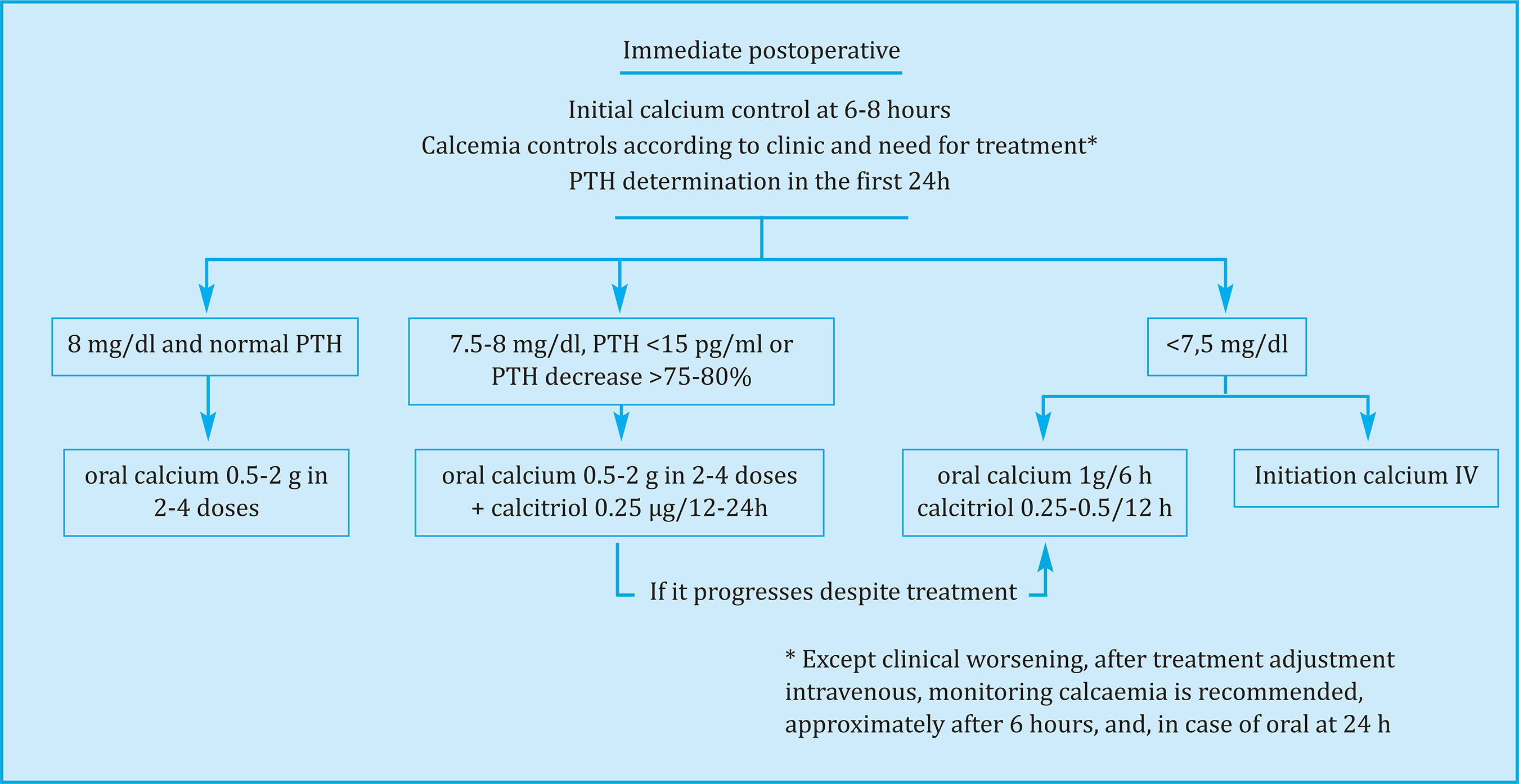
Initial follow-up of calcaemia and PTH
Assessing calcaemia and PTH in the first 6-8 hours after thyroidectomy and postoperative monitoring of serum total calcium (albumin corrected) or ionic calcium every 6-12 hours is required to diagnose and monitor postoperative hypoparathyroidism, which will be narrower in the patients at higher risk (Figure 2). The time interval for changes in calcium levels is longer than for PTH, and it may take 24-72 h after surgery for low calcium11. Postop calcium levels and variation have been used to establish instructive directions.
Ionic calcium levels (<1-1.1 mmol/l)23,24 and corrected serum calcium (generally <8 mg/dl)16,25,26 in the first 24 h postoperatively have been shown to predict hypocal-cemic development, although early PTH measurement is more sensitive and cost-effective25,27. The joint determination of PTH and calcaemia in the first 24 h postoperative period predicted the development of hypocalcaemia more precisely than each parameter in isolation16,27. The variation of total serum calcium in the first postoperative hours has been useful to predict the subsequent evolution: the neutral or positive trend of total calcium (no change or elevation between 2 consecutive postoperative measurements) predicted normocalcemia with a positive predictive value (PPV) 86-100%28-30. The negative trend (decrease) in total calcium was associated with the subsequent development of hypocalcemia28-30.
Since hungry bone syndrome is part of the differential diagnosis of postoperative hypocalcaemia, especially in patients with severe hyperparathyroidism or severe hyperthyroidism with high alkaline phosphatase levels, phosphorus determination may be very useful to differentiate this entity from hypocalcaemia due to hypoparathyroidism, since phosphorus levels will be decreased in the case of rapid remineralization of a bone subjected to hypermetabolism1,31,32.
If possible, taking turns in Trousseau's sign may be helpful in the postoperative period to identify clinical hypoparathyroidism and relative hypoparathyroidism.
Management of mild-moderate hypocalcemia with oral treatment
The general purpose of treatment is to keep blood glucose lower or slightly below the lower limit of the reference range1,32,33.
The calcium salt most commonly used for the correction of hypocalcemia is calcium carbonate because it contains more elemental calcium (40%) than calcium citrate (21%). Calcium citrate does not require gastric acidity for its absorption, therefore it can be more useful in patients with achlorhydria, low gastric acidity as observed in patients undergoing treatment with proton pump inhibitors, or patients with gastrectomy. The usual dose is 0.5-2 g of element calcium divided into 2-4 doses. The
optimal dose in terms of intestinal absorption seems to be 500 mg of element calcium per dose, since with higher doses a proportional increase in absorption is not achieved. The calcium salt should ideally be taken with meals to guarantee its best absorption and also act as a phosphorus chelator34-36.
Calcitriol is the active metabolite of vitamin D, which is why it has a rapid onset, increasing calcium absorption at the intestinal level. It is characterized by a shorter halflife (2-3 days) than ergocoleciferol or cholecalciferol (weeks), this being very useful because its effects are more quickly reversible in the case of iatrogenic hypercalcemia. Calcitriol can worsen hyperphosphatemia by increasing absorption of phosphates at the intestinal level. It is administered in doses of 0.25-2.0 μg/day. Occasionally, it is necessary to decrease the intake of phosphates in the diet due to the associated hyperphosphatemia, and phosphate binders can also be administered to decrease hyperphosphatemia in severe cases35,36.
Treatment of mild and moderate hypoparathyroidism is recommended to be carried out orally (Figure 2). In patients with PTH <15 pg/ml, or decrease in PTH level greater than 75-80% with respect to baseline, serum calcium <8.0 mg/dl or ionic calcium <1.0 mmol/l or <4.0 mg/dl measured within the first 6-8 hours postoperatively, it is recommended to start treatment with elemental calcium 0.5-2 g of element calcium divided into 2-4 doses with meals and calcitriol 0.25-0, 5 µg/day checking calcium and magnesium every 6-12 hours. In case of hypocalcemia progression despite previously described treatment or calcium less than 7.5 mg/dl, calcium should be increased to 1 g every 6 hours and calcitriol to 0.50-1 µg/day divided into twice a day. Also in these cases, intravenous calcium treatment may be necessary. Mild hypocalcemia (Ca >8.0 mg/dl) can be treated with oral calcium supplements37 in doses of 0.5-2 g of element calcium divided into 2-4 doses.
Since magnesium can decrease in hypocalcemia by inducing a decrease in PTH secretion and resistance to PTH activity, hypomagnesemia, in patients with normal renal function, should be supplemented with magnesium 400-1,000 mg/day, and, Furthermore, reducing constipation associated with high doses of calcium may
The administration of calcium salts of levothyroxine should be separated, because it inhibits its absorption. Levothyroxine is recommended to be taken 1 hour before or 3 hours after oral calcium salts1,31,32.
Recommendations:
- In the first 24 h after thyroidectomy, we suggest determining PTH levels and their percentage decrease with respect to preoperative values to detect those patients with the highest risk of hypocalcemia

- The available evidence does not allow us to recommend a specific cut-off point for PTH (absence of recommendation).
- After thyroidectomy, we recommend serial determination of ionic calcium or corrected total calcium to identify those patients with the highest risk of hypocalcemia, candidates for treatment with calcium and/or calcitriol supplements
 .
.- After thyroidectomy, we suggest determination of plasma phosphorus to identify and detect patients with possible hungry bone
 .
.- If possible, we suggest taking the Trousseau sign in turns
 .
.- We recommend orally treating mild and moderate hypoparathyroidism to keep blood glucose lower or slightly below the lower limit of the reference range
 .
.- We suggest treatment with elemental calcium 0.5-2 g divided into 2-4 doses, with meals and calcitriol 0.25-0.5 mg/day in patients with PTH <15 pg/ml, or decrease in level PTH greater than 75-80% with respect to baseline, or serum calcium <8.0 mg/dl or ionic calcium <1.0 mmol/l (or in mg/dl, ionic <4.0 mg/dl) measured within the first 6-8 h postoperatively, and follow-up with calcium and magnesium controls every 6-12 hours. In the event of hypocalcemia progression despite previously described treatment or calcium less than 7.5 mg/dl, we suggest increasing calcium to 1 g every 6 h and calcitriol to 0.50-1 µg/day divided twice by day and/or intravenous calcium
 .
.- We suggest the treatment of mild hypocalcemia (Ca >8.0 mg/dl) with oral calcium supplements in doses of 0.5-2 g in 2-4 doses
 .
.
Management of severe hypocalcemia
Treatment of severe hypocalcaemia, which presents with symptoms of carpopedal spasm, tetany, seizures or lengthening of the QT interval, or with a level <7.5 mg/dl, even if asymptomatic, is carried out with intravenous calcium.
Initially, treatment will be done with a bolus of 1 or 2 grams of calcium gluconate (GC) in 50 ml of 5% glucose serum or saline infused in 10-20 minutes. This dose raises the calcium level for about two or three hours, so it should be followed by a slow infusion of calcium in patients with persistent hypocalcemia (about 50 mg of element calcium per hour). This is achieved by adding 11 grams of GC = 11 ampoules of 10% GC, with 93 mg of element calcium per ampoule = 1,000 mg of element calcium → in 1,000 ml of 5% glucose serum or saline, to be administered at 50 ml /hour. Patients usually require 0.5 to 1.5 mg of calcium element/kg of body weight/hour. Doses should be adjusted to keep serum calcium below the normal limit11,36.
Rapid intravenous administration of calcium salts can cause vasodilation, decreased blood pressure, bradycardia, cardiac arrhythmias, syncope, and cardiac arrest. Patients receiving digoxin should be closely monitored for the risk of acute digitalis poisoning due to a probable induction of the positive inotropic action of digoxin. The infusion must not contain bicarbonate or phosphate, as they can form insoluble calcium salts. If these anions need to be perfused, an intravenous line must be used in another limb38,39. The use of GC against calcium chloride is recommended, since the latter can cause tissue necrosis if there is extravasation.
The infusion should be maintained until the patient receives an adequate oral calcium and vitamin D regimen that allows target levels to be maintained. For patients with hypoparathyroidism, calcitriol (dose of 0.25 to 0.5 µg twice a day) and oral calcium (3 to 4 grams of element calcium daily, divided into several doses) are recommended, which will be started together with intravenous infusion, stopping the infusion when the calcaemia reaches the lower limit of normality. Regarding treatment with recombinant human PTH (HRTH) in severe hypocalcae-mia due to acute hypoparathyroidism, there are very few published data. In an observational study carried out in 8 patients who were administered PTHrh for up to three weeks, a correction of hypocalcaemia was observed in 24 hours40. There are also some published cases of the use of HRTH in acute hypoparathyroidism, but without sufficient data to make a recommendation11,41,42. The THYPOS phase II study published in 2016 assessed its use in high-risk patients to prevent episodes of acute hypocalcemia and shorten hospital stay, with positive results43.
Recommendations:
- We suggest the use of intravenous calcium for the treatment of severe hypocalcemia
 .
.- We recommend the use of calcium gluconate versus calcium chloride due to the risk of necrosis in case of extravasation
 .
.- We suggest starting treatment with oral calcium and calcitriol together with intravenous calcium infusion
 .
.
Early and late postoperative period
Prophylactic guidelines for calcium and vitamin D supplementation after surgery may delay the recovery of parathyroids after surgical manipulation44, so we do not recommend their use, which is becoming less and less widespread. In the case of patients who require treatment at discharge, although the strategy of keeping calcium at the lower limit of normality in the first post-surgical month has been used, considering that it could be a stimulus for residual glandular tissue, we are not sure that a hypocalcemic environment is not in itself an attack on the glandular tissue, and further studies are necessary to conclude which level of calcaemia is optimal in the first month after surgery45. Patients requiring supplementation at discharge should be reevaluated after 1 or 2 weeks with a new test with determination of calcaemia and PTH, and if calcium levels are normal,
treatment will be reduced by approximately half, planning a subsequent reevaluation to try suspend it. It is important that the patient knows the symptoms of hypo and hypercalcemia so that they go to the emergency department if necessary, since discharge is frequent before the plasma calcium nadir is reached1.
Regarding the management of chronic hypoparathyroidism in the late postoperative period, treatment aims include: keeping the patient asymptomatic; maintain calcium levels close to the lower limit of normal but not exceed 0.5 mg/dl below it; prevent hypocalcemia; achieve a calcium-phosphorus product <55 mg2/dl2; and avoid hypercalciuria, hypercalcemia, and ectopic calcifications, including renal46. Treatment consists of supplementation with oral calcium and calcitriol and, after the latest guidelines, in which maintaining levels of 25 (OH) vitamin D >20 ng/ml is recommended, supplementing with cholecalciferol or ergocalciferol (the latter not available in Spain) if necessary. The use of thiazides can help control hypercalciuria. Phosphorus chelators may regulate this ion, although its use is only recommended for high levels (>6.5 mg/dl)36. Regarding the use of PTH analogues, studies carried out so far have shown that they stabilize plasma levels of calcium and phosphorus, significantly reducing the need for oral treatment. In 2015, the FDA (Foods and Drugs Administration) approved the use of rhPTH (1-84), along with calcium and vitamin D, to treat adults with poorly controlled hypoparathyroidism with conventional therapy, and in 2017 the European Commission did so47.
Recommendations:
Bibliografía
1 Orloff LA, Wiseman SM, Bernet VJ, Fahey TJ 3rd, Shaha AR, Shindo ML, et al. American Thyroid Association statement on postoperative hypoparathyroidism: diagnosis, prevention, and management in adults. Thyroid. 2018;28:830-41. [ Links ]
2 Lorente-Poch L, Sancho JJ, Muñoz-Nova JL, Sánchez-Velázquez P, Sitges-Serra A. Defining the syndromes of parathyroid failure after total thyroidectomy. Gland Surg. 2015;4:82-90. [ Links ]
3 Edafe O, Antakia R, Laskar N, Uttley L, Balasubramanian SP. Systematic review and meta-analysis of predictors of post-thyroidectomy hypocalcaemia. Br J Surg. 2014;101:307-20. [ Links ]
4 Kaya C, Tam AA, Dirikoç A, Kiliçyazgan A, Kiliç M, Türkölmez $, et al. Hypocalcemia development in patients operated for primary hyperparathyroidism: Can it be predicted preoperatively? Arch Endocrinol Metab. 2016;60:65-471. [ Links ]
5 Erbil Y, Ozbey NC, Sari S, Unalp HR, Agcaoglu O, Ersoz F, et al. Determinants of postoperative hypocalcemia in vitamin D-deficient Graves' patients after total thyroidectomy. Am J Surg. 2011; 201:685-91. [ Links ]
6 Kirkby-Bott J, Markogiannakis H, Skandarajah A, Cowan M, Fleming B, Palazzo F. Preoperative vitamin D deficiency predicts postoperative hypocalcemia after total thyroidectomy. World J Surg. 2011;35:324-30. [ Links ]
7 Erbil Y, Barbaros U, Temel B, Turkoglu U, Işsever H, Bozbora A, et al. The impact of age, vitamin D(3) level, and incidental parathyroidectomy on postoperative hypocalcemia after total or near total thyroidectomy. Am J Surg. 2009;197:439-46. [ Links ]
8 Kaderli RP, Riss P, Dunkler D, Pietschmann P, Selberherr A, Scheuba C, et al. The impact of vitamin D status on hungry bone syndrome after surgery for primary hyperparathyroidism. Eur J Endocrinol. 2018; 178:1-9. [ Links ]
9 Rolighed L, Rejnmark L, Sikjaer T, Heickendorff L, Vestergaard P, Mosekilde L, et al. Vitamin D treatment in primary hyperparathyroidism: a randomized placebo controlled trial. J Clin Endocrinol Metab. 2014;99:1072-80. [ Links ]
10 Grey A, Lucas J, Horne A, Gamble G, Davidson JS, Reid IR. Vitamin D repletion in patients with primary hyperparathyroidism and coexistent vitamin D insufficiency. J Clin Endocrinol Metab. 2005;90:2122-6. [ Links ]
11 Stack BC, Bimston DN, Bodenner DL, Brett EM, Dralle H, Orloff LA, et al. American association of Clinical Endocrinologists and American College of Endocrinology disease state clinical review: postoperative hypoparathyroidismo- definitions and management. Endocr Pract. 2015;21:674-85. [ Links ]
12 McLeod IK, Arciero C, Noordzij JP, Stojadinovic A, Peoples G, Melder PC, et al. The use of rapid parathyroid hormone assay in predicting postoperative hypocalcemia after total or completion thyroidectomy. Thyroid. 2006;16:259-65. [ Links ]
13 Scurry WC, Beus KS, Hollenbeak CS, Stack BC. Perioperative parathyroid hormone assay for diagnosis and management of postthyroidectomy hypocalcemia. Laryngoscope. 2005; 115:1362-6. [ Links ]
14 Alía P, Moreno P, Rigo R, Francos J-M, Navarro M-A. Postresection parathyroid hormone and parathyroid hormone decline accurately predict hypocalcemia after thyroidectomy. Am J Clin Pathol. 2007;127:592-7. [ Links ]
15 Castro A, Del Rio L, Gavilan J. Stratifying the risk of developing clinical hypocalcemia after thyroidectomy with parathyroid hormone. Otolaryngol Head Neck Surg. 2018;158:76-82. [ Links ]
16 Asari R, Passler C, Kaczirek K, Scheuba C, Niederle B. Hypoparathyroidism after total thyroidectomy: a prospective study. Arch Surg. 2008;143:132-7. [ Links ]
17 Youngwirth L, Benavidez J, Sippel R, Chen H. Parathyroid hormone deficiency after total thyroidectomy: incidence and time. J Surg Res. 2010;163:69-71. [ Links ]
18 Kim JP, Park JJ, Son HY, Kim RB, Kim HY, Woo SH. Effectiveness of an i-PTH measurement in predicting post thyroidectomy hypocalcemia: prospective controlled study. Yonsei Med J. 2013;54:637-42. [ Links ]
19 Kala F, Sarici IS, Ulutas KT, Sevim Y, Dogu A, Sarigoz T, et al. Intact parathormone measurement 1 hour after total thyroidectomy as a predictor of symptomatic hypocalcemia. Int J Clin Exp Med. 2015;8:18813-8. [ Links ]
20 Lombardi CP, Raffaelli M, Princi P, Santini S, Boscherini M, De Crea C, et al. Early prediction of postthyroidectomy hypocalcemia by one single iPTH measurement. Surgery. 2004;136:1236-41. [ Links ]
21 Del Río L, Castro A, Bernáldez R, Del Palacio A, Giráldez CV, Lecumberri B, et al. Parathyroid hormone as a predictor of post-thyroidectomy hypocalcemia. Acta Otorrinolaringol Esp. 2011;62:265-73. [ Links ]
22 Lifante J-C, Payet C, Ménégaux F, Sebag F, Kraimps J-L, Peix J-L, et al. Can we consider immediate complications after thyroidectomy as a quality metric of operation? Surgery. 2017;161:156-65. [ Links ]
23 Rosa KM, de Matos LL, Cernea CR, Brandão LG, de Araújo Filho VJF. Postoperative calcium levels as a diagnostic measure for hypoparathyroidism after total thyroidectomy. Arch Endocrinol Metab. 2015;59(5): 428-33. [ Links ]
24 de Andrade Sousa A, Salles JMP, Soares JMA, de Moraes GM, Carvalho JR, Rocha PRS. Course of ionized calcium after thyroidectomy. World J Surg. 2010;34:987-92. [ Links ]
25 Lo CY, Luk JM, Tam SC. Applicability of intraoperative parathyroid hormone assay during thyroidectomy. Ann Surg. 2002; 236:564-9. [ Links ]
26 Lindblom P, Westerdahl J, Bergenfelz A. Low parathyroid hormone levels after thyroid surgery: a feasible predictor of hypocalcemia. Surgery. 2002;131:515-20. [ Links ]
27 Graff AT, Miller FR, Roehm CE, Prihoda TJ. Predicting hypocalcemia after total thyroidectomy: parathyroid hormone level vs. serial calcium levels. Ear Nose Throat J. 2010;89:462-5. [ Links ]
28 Luu Q, Andersen PE, Adams J, Wax MK, Cohen JI. The predictive value of perioperative calcium levels after thyroid/parathy-roid surgery. Head Neck. 2002;24:63-7. [ Links ]
29 Güllüoğlu BM, Manukyan MN, Cingi A, Yeğen C, Yalin R, Aktan AO. Early prediction of nor-mocalcemia after thyroid surgery. World J Surg. 2005;29:1288-93. [ Links ]
30 Husein M, Hier MP, Al-Abdulhadi K, Black M. Predicting calcium status post thyroidectomy with early calcium levels. Otolaryngol Head Neck Surg. 2002;127:289-93. [ Links ]
31 Kakava K, Tournis S, Papadakis G, Karelas I, Stampouloglou P, Kassi E, et al. Postsurgical hypoparathyroidism: a systematic review. In Vivo. 2016;30(3):171-9. [ Links ]
32 Kazaure HS, Sosa JA. Surgical Hypoparathyroidism. Endocrinol Metab Clin North Am. 2018;47(4):783-96. [ Links ]
33 Walker Harris V, Jan De Beur S. Postoperative hypoparathyroidism: medical and surgical therapeutic options. Thyroid. 2009; 19:967-73. [ Links ]
34 Gafni RI, Collins MT. Hypoparathyroidism. N Engl J Med. 2019;380(18):1738-47. [ Links ]
35 Tecilazich F, Formenti AM, Frara S, Giubbini R, Giustina A. Treatment of hypoparathyroidism. Best Pract Res Clin Endocrinol Metab. 2018;32:955-64. [ Links ]
36 Brandi ML, Bilezikian JP, Shoback D, Bouillon R, Clarke BL, Thakker RV, et al. Management of hypoparathyroidism: summary statement and guidelines. J Clin Endocrinol Metab. 2016;101:2273-83. [ Links ]
37 Calvo Espino P, Rivera Bautista JÁ, Artés Caselles M, Serrano González J, García Pavía A, García-Oria MJ, et al. Serum levels of intact parathyroid hormone on the first day after total thyroidectomy as predictor of permanent hypoparathyroidism. Endocrinol Diabetes Nutr. 2019;66:195-201. [ Links ]
38 Tohme JF, Bilezikian JP. Diagnosis and treatment of hypocalcemic emergencies. The Endocrinologist. 1996;6:10-8. [ Links ]
39 https://cima.aemps.es/cima/pdfs/es/ft/69465/FT_69465.pdf. [ Links ]
40 Shah M, Bancos I, Thompson GB, Richards ML, Kasperbauer JL, Clarke BL, et al. Teriparatide therapy and reduced postoperative hospitalization for postsurgical hypoparathyroidism. JAMA Otolaryngol Neck Surg. 2015;141(9): 822-7. [ Links ]
41 Mishra PE, Schwartz BL, Sarafoglou K, Hook K, Kim Y, Petryk A. Short-term PTH(1-34) therapy in children to correct severe hypocalcemia and hyperphosphatemia due to hypoparathyroidism: two case studies. Case Rep Endocrinol. 2016;2016:1-4. [ Links ]
42 Andrysiak-Mamos E, Żochowska E, Kaźmierczyk-Puchalska A, Popow M, Kaczmarska-Turek D, Pachucki J, et al. Treatment of severe life threatening hypocalcemia with recombinant human teriparatide in patients with postoperative hypoparathyroidism - a case series. Endokrynol Pol. 2016;67:403-12. [ Links ]
43 Palermo A, Mangiameli G, Tabacco G, Longo F, Pedone C, Briganti SI, et al. PTH(1-34) for the primary prevention of postthyroidectomy hypocalcemia: the THYPOS trial. J Clin Endocrinol Metab. 2016;101:4039-45. [ Links ]
44 Dedivitis RA, Aires FT, Cernea CR. Hypoparathyroidism after thyroidectomy: prevention, assessment and management. Curr Opin Otolaryngol Head Neck Surg. 2017;25:142-6. [ Links ]
45 Sitges-Serra A, Gómez J, Barczynski M, Lorente-Poch L, Iacobone M, Sancho J. A nomogram to predict the likelihood of permanent hypoparathyroidism after total thyroidectomy based on delayed serum calcium and iPTH measurements. Gland Surg. 2017; 6(Suppl 1):S11-9. [ Links ]
46 Bollerslev J, Rejnmark L, Marcocci C, Sho-back DM, Sitges-Serra A, van Biesen W, et al. European Society of Endocrinology Clinical Guideline: treatment of chronic hypoparathyroidism in adults. Eur J Endocrinol. 2015;173:G1-20. [ Links ]
47 Mannstadt M, Bilezikian JP, Thakker RV, Hannan FM, Clarke BL, Rejnmark L, et al. Hypoparathyroidism. Nat Rev Dis Primers. 2017;3:17080. [ Links ]
Received: February 23, 2020; Accepted: June 21, 2020











 text in
text in