My SciELO
Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista de Osteoporosis y Metabolismo Mineral
On-line version ISSN 2173-2345Print version ISSN 1889-836X
Rev Osteoporos Metab Miner vol.14 n.1 Madrid Jan./Mar. 2022 Epub Aug 22, 2022
https://dx.doi.org/10.4321/s1889-836x2022000100003
ORIGINALS
Clinical practice guidelines for postmenopausal, glucocorticoid-induced and male osteoporosis: 2022 update
1aInternal Medicine Service. Marqués de Valdecilla University Hospital. Santander (Spain)
1bDepartment of Medicine and Psychiatry. University of Cantabria. IDIVAL. Santander (Spain)
2aRheumatology Service. Hospital Clinic. Barcelona (Spain)
2b University of Barcelona, IDIBAPS, CIBERehd. Barcelona (Spain)
3Department of Medicine and Psychiatry. University of Cantabria, IDIVAL. Santander (Spain)
4aInternal Medicine Service. Rio Hortega University Hospital. Valladolid (Spain)
4bDepartment of Medicine. University of Valladolid. Valladolid (Spain)
This update incorporates the most relevant information that has emerged during the seven years since the publication of the previous version, with a particular focus on diagnostic procedures and therapeutic options. Among the diagnostic procedures, we highlight the use of the Trabecular Bone Score (TBS) and densitometry for identifying the risk of vertebral fractures. Novel therapeutic modalities include the use of anabolic drugs with comparative studies focused on their efficacy for the treatment of severe osteoporosis. Guidelines for actions to be taken after discontinuation of antiresorptive agents, sequential therapy and current recommended treatment schemes are included
Key words: osteoporosis; fractures; densitometry; anabolic; antiresorptive
Introduction
Seven years have passed since the publication of the previous version of the Osteoporosis Guidelines of the Spanish Society for Bone Research and Mineral Metabolism (SEIOMM) that was created in accordance with the standard methodology of evidence-based medicine1. This update incorporates the most essential information that has appeared since the publication of the previous version, with particular reference to new diagnostic procedures and therapeutic options. Novel diagnostic modalities discussed in these guidelines include the Trabecular Bone Score (TBS) and the detection of vertebral fractures by densitometry. Among the therapeutic options, we discuss the use of novel anabolic drugs (abaloparatide and romosozumab). Studies that compare the efficacy of various drug regimens for the treatment of severe osteoporosis are also considered. Likewise, the guidelines for action after the withdrawal of antiresorptive drugs and other sequential and combined treatment schemes are assessed.
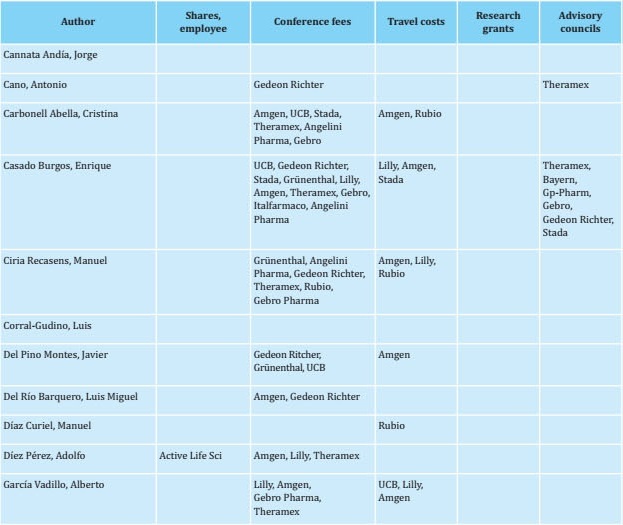
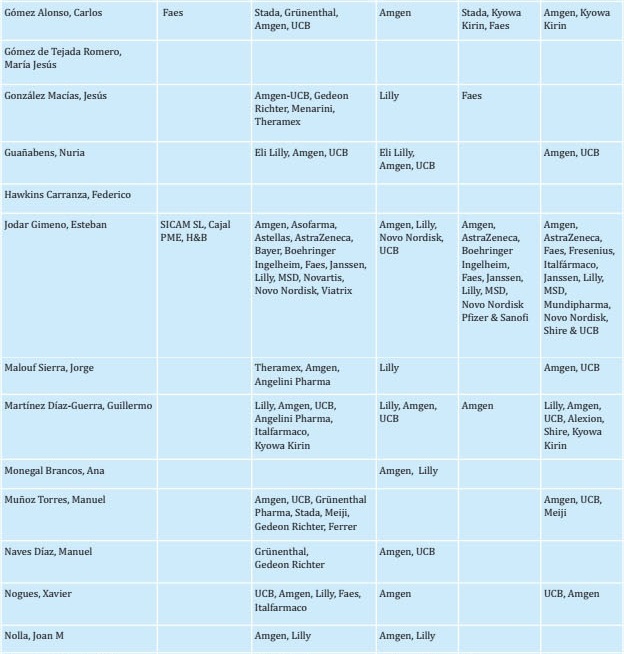
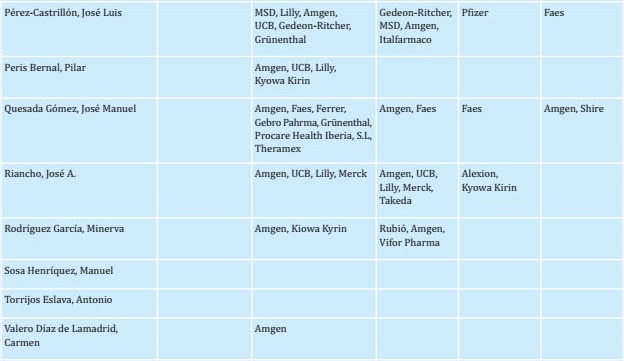
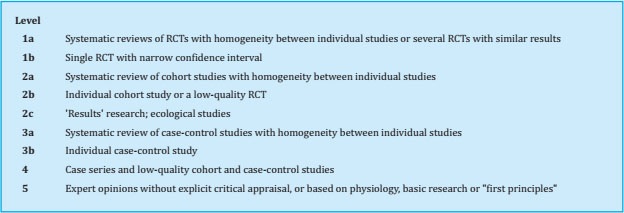
To prepare this update, a group of experts (see author listing) reviewed each of the sections and incorporated new findings from reports published in recent years. The initial draft of the manuscript was then critically examined by a group of experts. Once their comments were considered, the new text was distributed to other interested parties, including SEIOMM partners, patient associations, the Spanish Agency for Medicines and Health Products, and pharmaceutical industries so that each might provide additional comments and contributions to the document. The document was then re-analyzed again by the group of experts tasked with drafting the guidelines. The recommendations were graded according to the level of evidence as indicated in Tables S1 and S2.
The topics reviewed in this document include (1) diagnostic and therapeutic aspects of primary osteoporosis in postmenopausal women, (2) specific findings associated with osteoporosis in males, and (3) new information on the diagnosis and treatment of glucocorticoid-induced osteoporosis.
ASSESSMENT OF PATIENTS AT RISK FOR OSTEOPOROSIS
1. Fracture risk factors
The main factors associated with the risk of bone fractures in patients presenting with osteoporosis include gender, age, bone mineral density (BMD), history of fragility fracture, history of hip fracture in a first-degree relative, and low body weight (i.e., body mass index [BMI] <20 kg/m2). Paradoxically, obesity can also be a risk factor for some peripheral fractures, including those of the humerus and distal third of the radius. Recognised risk factors also include various diseases including hypogonadism, early menopause, prolonged amenorrhea, anorexia nervosa, malabsorption, rheumatoid arthritis, diabetes (particularly type 1), immobilization, as well as their treatments, e.g., glucocorticoids, inhibitors of aromatase or gonado-tropin-releasing hormone agonists2,3. Other disorders and medications that may be associated with the development of osteoporosis, (although probably less strongly) are hyperparathyroidism, hyperthyroidism, selective serotonin reuptake inhibitors, proton pump inhibitors, and anticonvulsants, as well as smoking and excessive alcohol consumption. Calcium deficiency and vitamin D deficiency have traditionally been considered risk factors for osteoporosis, although their precise role continues to be a subject of debate (Table 1).
Factors associated with an increased risk of falls, including postural instability, inability to get up from a chair, visual impairment, and some neurological problems are also associated with an increased risk of fractures.
After a first fracture, the greatest risk of sustaining a new fracture occurs within the first two years, particularly if the first fracture was vertebral4-6. This has led to the concept of an "imminent risk" of fracture. The main factors that have been associated with imminent risk are older age, female gender, white race, recent fracture, falls, and some comorbidities and treatments (e.g., very low bone mass, cardiovascular disease, obstructive pulmonary disease, chronic and depression, and anxiety, as well as the use of sedatives, hypnotics, glucocorticoids, and muscle relaxants).
In conclusion, recent evidence suggests that an assessment of clinical risk factors combined with the measurement of BMD is an effective method for assessing fracture risk (Recommendation A).
2. Bone densitometry and related techniques
Dual-energy X-ray absorptiometry (DXA) can be used to quantify BMD and is thus the procedure most commonly used to estimate fracture risk7. The results are expressed in terms of T-score, which is the number of standard deviations (SDs) by which the BMD value obtained differs from that of the normal young adult population (i.e., 20–29 years of age). The World Health Organization (WHO) guidelines state that osteoporosis can be diagnosed when the BMD is less than -2.5 T8. The organization has since clarified that this value must correspond to a measurement made on the neck of the femur using data from the National Health and Nutrition Examination Survey (NHA-NES III) study as a reference9. By contrast, the International Society for Clinical Densitometry (ISCD)10 states that this diagnosis can be established based on a -2.5 T value detected in the lumbar spine or total hip as well as the femoral neck. The WHO also defined normal bone density, osteopenia (i.e., low bone mass), as well as established or severe osteoporosis (Table 2).
Table 2. WHO diagnostic criteria for osteoporosis

BMD: bone mineral density; T (T-score or T index): comparison with the BMD value reached in a young reference population.
BMD measured at the mid-third of the radius may also be used to diagnose osteoporosis when the hip and lumbar spine cannot be used or interpreted11.
In, The ISCD recommends that instead of T-scores, Z-scores adjusted for ethnicity or race be used when diagnosing osteoporosis in premenopausal women, men younger than 50 years of age, and children. Z-scores ≤-2.0 are identified as "low bone mineral density for age chronological" or "below expected range for age". Z-scores >-2.0 are identified as"within expected range for age".
Evaluation of therapeutic efficacy is an indication for densitometry. This examination might be repeated after two to three years of treatment.
Other measurement techniques, including quantitative ultrasonometry and quantitative computed tomography, among others, also provide values that are related to fracture risk. However, they are not recommended as diagnostic procedures at this time.
Lateral projections of DXA studies can be used to identify vertebral fractures (i.e., VFA, or “vertebral fracture assessment”). However, the accuracy of this procedure is lower than that of conventional radiography, most notably for the diagnosis of fractures of the upper thoracic vertebrae.
The Trabecular Bone Score (TBS) is a parameter that describes bone texture based on data obtained from a DXA image of the lumbar spine. TBSs are typically reduced in patients who have sustained fragility fractures, and it is a useful value for assessing fracture risk in women and men over 50 years of age, independent of BMD findings. The combination of BMD and TBS is superior to BMD alone for the prediction of fracture risk. A TBS may be particularly useful in assessing fracture risk in patients diagnosed with diabetes or primary hyperparathyroidism as well as those treated with glucocorticoids. The TBS is also expressed in absolute terms and as a T- score.
A TBS value <1.230 (T <-3) is indicative of a degraded trabecular microstructure and a high risk of fracture. The TBS has been included in the Fracture Risk Assessment Tool (FRAX) which can be used to calculate the absolute risk of fracture in a given patient.
Despite the proven usefulness of DXA for assessing patients with an elevated risk of sustaining a fracture, the sensitivity and specificity of this modality remain limited. DXA does not identify all subjects at risk of fracture; more than 50% of peripheral fractures occur in patients with a T-score >-2.512,13. Current trends suggest that BMD measurements might be considered together with the clinical risk factors when calculating an absolute fracture risk14,15.
There are no universally accepted criteria regarding when to perform densitometry. The general recommendation is that this procedure might be performed when risk factors that are strongly associated with osteoporosis or fractures emerge (Table 1), including:
Disorders frequently associated with osteoporosis, such as rheumatoid arthritis, early menopause, hyperparathyroidism, hyperthyroidism, malabsorption, and anorexia nervosa, among others.
Treatments with negative effects on the bone, such as glucocorticoids, antiestrogens, and antiandrogens, among others.
Other factors (especially if two of them are observed in a single patient): age over 65 years (according to some authors), low weight (BMI <20 kg/m2), family history of osteoporosis, alcoholism, and smoking, among others.
In conclusion, DXA can be used to measure BMD in the proximal femur and lumbar spine to assess the risk of fracture (Recommendation A). A TBS can provide additional information on the risk of fracture in an individual patient (Recommendation B).
3. Markers of bone turnover
Bone turnover markers (BTMs) provide information on the dynamics of bone turnover. Among the markers of bone formation, significant research has focused on levels of osteocalcin, bone alkaline phosphatase, and the carboxy –and amino-terminal propeptides of type I procollagen (PICP and P1NP). Markers of bone resorption include the carboxy– and amino-terminal telopeptides of collagen I (CTX in blood, s-CTX and NTX in urine) and tartrate-resistant acid phosphatase 5b (FATR 5b). Various international organizations (for example, the International Federation of Clinical Chemistry) have recommended the use of P1NP and s-CTX as markers of bone formation and resorption, respectively, for ongoing and future clinical studies. It is important to control the variability of these measurements by obtaining biological samples consistently between 08:00 and 10:00 hrs after an overnight fast.
While BTMs are not useful for diagnosing osteoporosis, this information may be combined with other risk factors to identify, patients with a higher risk of sustaining a fracture. These values are particularly useful for the early assessment of responses to both antiresorptive and anabolic therapy (Evidence 2a)16,17. For example, measurements of s-CTX and PINP are recommended as an effective means to monitor bone turnover after discontinuation of denosumab18.
In conclusion, BTMs can be useful for evaluating therapeutic responses (Recommendation B), but they must be measured under standardised conditions. They are not used routinely to diagnose osteoporosis.
4. Identification of vertebral fractures
Conventional radiography is not sufficiently sensitive or specific when used to assess changes in bone mass8. However, the use of this modality is essential when attempting to identify fractures.
A diagnosis of a vertebral fracture requires a decrease of at least 20–25% in height19. This is because slight wedging can be confused with deformities of another origin (e.g., sequelae of Scheuermann's disease, small wedging of a degenerative type)20. Thus, VFA by DXA may be useful as a first step. Spinal radiography (or DXA) is recommended for patients over the age of 70 years with suspected osteoporosis who present with back pain, glucocorticoid treatment, or a significant decrease in height (>4 cm based on historical data or >2 cm in confirmed height)21.
In conclusion, reliable identification of vertebral fractures is important in decision-making because these lesions represent a risk for future fractures. Evaluation can be done by radiography or by VFA. However, radiography should not be used as a method of assessing bone mass to establish a diagnosis of osteoporosis (Recommendation A).
5. Study protocol
In addition to anamnesis and a physical examination, an evaluation of a patient with suspected osteoporosis should include a complete blood count and determination of basic biochemical parameters (kidney and liver function and serum levels of calcium, albumin, phosphorus, alkaline phosphatase, thyrotropin (TSH), and 25-hydroxyvitamin D, as well as a serum protein electrophoresis study). It is useful to quantify calciuria. These tests should be performed before starting treatment and then repeated if clinically indicated. The usefulness of parathyroid hormone (PTH) levels and BTMs remains controversial (see the previous section). Bone densitometry and an assessment of potential vertebral fractures by VFA or radiology will almost always be required. Pertinent studies should be performed to rule out secondary causes of osteoporosis (e.g., hypercortisolism, celiac disease, and systemic mastocytosis, among others) in younger patients (Recommendation C).
6. Risk prediction tools
Various scoring scales have been developed to assess either the risk of developing osteoporosis (i.e., low DXA), or sustaining osteoporotic fractures. Current scoring scales used to assess the risk of densitometric osteoporosis do not include BMD but are useful in deciding when densitometry evaluations should be performed.
The simplest method, known as the Osteoporosis Self-assessment Tool [OST])22,23 includes only patient age and weight which are variables included in all assessment strategies.
To assess the risk of fractures, the addition of findings from DXA to the clinical data results in their improved predictive value. Several instruments have been developed for this purpose, including FRAX24, the Garvan Medical Research Institute scale25, and the QFracture Index26. All three have similar discriminatory capacities albeit with only moderate performance27,28. FRAX is the most widely used of these instruments on a worldwide basis. Unfortunately, its adaptation in Spain has been inadequate29 and it underestimates the risk of fracture, most notably major osteoporotic fractures. Other tools, such as EPIC, which has been adjusted to the Spanish population, are currently undergoing validation.
In conclusion, although fracture risk prediction tools may be helpful in decision-making in some cases, their predictive value for our population is limited. Adaptations of FRAX may be used with caution pending the development and validation of newer and more precise instruments (Recommendation C).
AVAILABLE TREATMENTS FOR POSTMENOPAUSAL OSTEOPOROSIS
1. Non-pharmacological interventions
A balanced diet should be maintained by all patients diagnosed with postmenopausal osteoporosis. This would include a protein intake of 1–1.5 g/kg/day. While sun exposure will promote essential vitamin D synthesis, additional supplementation may be needed (see below)30. Furthermore, recent evidence suggests that physical exercise that loads the skeleton has a positive effect with respect to preventing falls and reducing the risk of fracture31. Routine exercise is recommended, for example, walking every day for at least 30 minutes.
Smoking and excessive alcohol consumption should be avoided, as both are factors associated with decreased bone mass and an increased risk of fractures32,33.
Although the efficacy of fall prevention programs (beyond basic physical exercise) remains controversial, recent evidence suggests that they are useful in institutionalised elderly patients who undergo repeated falls34,35. Hip protectors are slightly effective at reducing the risk of hip fracture. However, poor tolerance by some patients, poor adherence, and a slight increase in the risk of pelvic fractures limit its application36.
2. Calcium and vitamin D
Patients treated with antiresorptive or anabolic drugs for osteoporosis should be certain to maintain an adequate intake of calcium and vitamin D37,38. Serum levels of 25-hydroxyvitamin D (25(OH)D) should be maintained above 20–25 ng/ml, preferably above 30 ng/ml39. The recommended daily dose of vitamin D is generally between 800–1200 IU/day, although some patients may need higher doses to maintain adequate serum levels of 25(OH)D. While bi-weekly or monthly equivalents can be considered, administration of large amounts of vitamin D in a single dose (e. g., 500,000 IU/year)40 is not recommended. The standard dose of calcifediol (25(OH)D3) is 0.266 micrograms every 15–30 days. This form of vitamin D may be preferable in patients with advanced liver disease or problems with intestinal absorption. Occasionally, these patients may require parenteral administration.
Daily intake of calcium should be maintained at 1000-1200 mg/day30. While it is preferable to obtain this amount from dietary sources, supplements can be added as necessary. The general population, particularly the elderly, should be advised to maintain adequate nutrient intake, including appropriate levels of calcium and vitamin D. However, the isolated effects of calcium and vitamin D on the progression of osteoporosis are not well-understood; if they exist at all, their impact seems to be limited41-43.
In conclusion, patients at risk for developing osteoporosis and those undergoing treatment with antiresorptive or anabolic drugs should receive and be certain that they are taking in an adequate supply of calcium and vitamin D. However, these nutrients alone are insufficient treatments in patients who have developed osteoporosis (Recommendation A).
3. Calcitonin
Although treatment with calcitonin was associated with a slight reduction in the risk of vertebral fractures, it has no impact on the risk of peripheral fractures. Furthermore, long-term calcitonin use has been associated with an increased risk of tumors. Thus, calcitonin is not approved for the treatment of osteoporosis44,45.
4. Thiazides
Although numerous observational studies suggested that treatment with thiazides resulted in increased bone mass and a concomitant reduction in the risk of fracture46, we have no data that can be construed as recommending its use as a treatment for osteoporosis. Thiazide treatment (e. g., 12–50 mg/day of hydrochlorothiazide or chlortha-lidone) can be considered for patients presenting with hypercalciuria47 (Recommendation D).
5. Estrogen therapy
The results of several clinical trials have revealed the efficacy of estrogens for the prevention of fractures. A recent network meta-analysis revealed that estrogen therapy (with or without progesterone) reduced the risk of vertebral fracture by 34% (hazard ratio [HR], 0.66; 95% confidence interval [CI], 0.49–0.89); hip fracture by 29% (HR, 0.71; 95% CI, 0.52–0.98); and non-vertebral fractures by 21% (HR, 0.79; 95% CI, 0.70-0.90)48. However, the side effects of estrogen therapy revealed by the Women’s Health Initiative (WHI) study and other trials include an increase in cardiovascular events and breast cancer. Thus, estrogen is not recommended as a treatment for osteoporosis ex cept in women with early menopause or at a high risk of fracture in which there is no other therapeutic option available49. Estrogens may be an effective treatment for osteoporosis in women already receiving these drugs as therapy for the climacteric syndrome.
In conclusion, although estrogen therapy is effective in preventing osteoporotic fractures, it is not recommended for routine use given the possibility of serious side effects (Recommendation A). Estrogens can be considered in patients exhibiting early menopause who have no other contraindications and/or in cases in which no other therapeutic options are available (Recommendation D).
6. Selective Estrogen Receptor Modulators (SERMs)
Results from several recent studies document that these drugs can increase BMD in the spine over follow-up periods as long as eight years50,51. A recent meta-analysis revealed that raloxifene and bazedoxifene reduce the risk of vertebral fracture by 40%, although neither drug has any impact on non-vertebral fractures48. The main complication associated with this class of drugs is an increased risk of venous thromboembolic disease.
In conclusion, SERMs may be indicated for the treatment of osteoporosis because they reduce vertebral fractures, but they do not reduce the risk of non-vertebral fractures (Recommendation A).
7. Tibolone
Although the use of this drug will reduce the risk of both vertebral and non-vertebral fractures in women under 60 years of age (or <10 years of menopause)52,53, its cardiovascular side effects limit its use. At this time, tibolone may be prescribed for patients who are not at high risk for cardiovascular disease or breast cancer who cannot be treated with other drugs (Recommendation B). This drug has not been approved for the treatment of osteoporosis in Spain.
8. Phytoestrogens and isoflavones
Isoflavones may have a favorable effect on BMD54. However, they are not currently recommended for the treatment of osteoporosis due to the lack of data focused on their efficacy in preventing fractures.
9. Bisphosphonates (BPs)
9.1. Etidronate
While etidronate reduces the incidence of vertebral fractures by about 40%55, it has no impact on non-vertebral fractures (Evidence 1a; Recommendation A). This drug has fallen into disuse as more effective BPs have become available.
9.2. Alendronate
Alendronate increases BMD at the lumbar spine and the hip in both treatment and prevention studies performed in osteoporotic women (Evidence 1a). Both daily and weekly administration of this drug result in similar efficacy (Evidence 1a). At a dose of 70 mg/week, alendronate reduces the incidence of vertebral, non-vertebral, and hip fractures by ~45%, 25–30%, and 45–55%, respectively56,57 (Evidence 1a). Most clinical trials focused on this drug included a treatment period of three to five years. However, administration over longer periods may sometimes be recommended. One extension study revealed that patients who discontinued treatment after five years had a higher risk of suffering clinical vertebral fractures than those who continued on this drug58. Older patients with low BMDs at the femoral neck at the time of treatment withdrawal exhibit a greater risk of fracture, including non-vertebral fractures59,60. Several meta-analyses and studies with data from real-world practice documented efficacy findings that were similar to those reported previously48,61. Alendronate is generally well tolerated, although it can result in some side effects (described below). Long-term use of this drug has been associated with an increase in atypical fractures. Recently, there has been speculation as to its possible beneficial cardiovascular ef fects62.
In conclusion, alendronate has a definitive role in the treatment of osteoporosis as it reduces the risk of vertebral, non-vertebral, and hip fractures in susceptible individuals (Recommendation A).
9.3. Risedronate
A recent systemic review and network meta-analysis documented the efficacy of risedronate in preventing vertebral, non-vertebral, and hip fractures in postmenopausal women with osteoporosis or osteopenia. The reduction in risk of fracture compared to placebo was 39% for vertebral fracture, 27% for hip fracture, and 22% for non-vertebral fractures48,63 (Evidence 1a). Risedronate can be administered in single doses of 35 mg per week or 75 mg on two consecutive days per month64,65. A new gastro-resistant formulation has been developed that does not require fasting before its administration64. Risedronate is well tolerated with side effects similar to those of other BPs as described below.
In conclusion, administration of risedronate results in reductions in the incidence of vertebral, non-vertebral, and hip fractures. Thus, this drug has a definite role in the treatment of osteoporosis (Recommendation A).
9.4 Ibandronate
Ibandronate can be administered orally at 150 mg/dose once a month or intravenously at 3 mg every 3 months intravenously (NB: the intravenous formulation is not marketed in Spain). Ibandronate reduces the risk of vertebral fractures by ~60% but has no impact on non-vertebral fractures (Evidence 1b). In a meta-analysis that included 107 trials focused on drugs that can be used to treat osteoporosis, ibandronate was identified as somewhat less efficacious at reducing the incidence of fractures than other BPs48.
In conclusion, ibandronate reduces the risk of vertebral fractures (Recommendation A), although had no apparent effect on non-vertebral fractures.
9.5. Zoledronate
Zoledronate administered intravenously at a dose of 5 mg/year reduces the incidence of vertebral, non-vertebral, and hip fractures by 70%, 25%, and 40%, respectively66 (Evidence 1b). Patients who, continue treatment with zoledronate for an additional three years after completion of an initial three years of treatment benefit from an additional 50% reduction in the risk of vertebral fracture compared to those who are not maintained on this regimen67. In a clinical trial that included women with what was called “osteopenia” who were older than 65 years of age, administration of this drug every 18 months also reduced the incidence of vertebral and non-vertebral fractures68. The side effects of this drug are described in the section to follow. While one network meta-analysis identified no differences between zoledronate and any of the other BPs studied in terms of fracture prevention69, two other studies revealed that zoledronate was shown to be more effective than the other formulations70,71.
In conclusion, zoledronate also reduces the incidence of vertebral, non-vertebral, and hip fractures, and thus plays an important role in osteoporosis treatment (Recommendation A).
9.6. Adverse effects of bisphosphonates72,73
BPs are generally safe and well-tolerated drugs. However, given their central role in the treatment of osteoporosis, possible adverse effects are discussed in detail below. It should be noted that other beneficial effects of these drugs have been described, including a decrease in mortality, especially that associated with cardiovascular events, and a reduction in the incidence of some cancers. However, the actual extent of these effects remains controversial74-76.
Adverse effects on the upper digestive tract have been described in patients taking oral BPs (i.e., esophagitis and esophageal ulcers). These responses can be largely avoided if the drug is ingested with a glass of water with an upright position maintained for the following 30–60 minutes. Contrary to what was suggested in some of the initial studies, these drugs do not increase the incidence of cancer of the esophagus or stomach77,78. However, BPs should not be prescribed for patients with disorders of the upper digestive tract, notably those with difficulty swallowing or Barrett's esophagus.
Acute-phase response or flu-like symptoms have been described mainly in response to intravenous BPs. This reaction typically appears within 24–36 hours of drug administration, can be relieved with acetaminophen, and usually disappears within three days79. This response had been reported in 25–35% of patients receiving intravenous zoledronate for the first time. The intensity typically diminishes in response to subsequent injections.
Studies regarding the association of BP treatment (especially intravenous) with atrial fibrillation have led to discordant results80. This has not been identified as a potential limitation for treatment in cases in which these drugs are indicated. Of note, several studies documented a reduced incidence of cardiovascular events in patients treated with BPs81,82.
BPs are not recommended in patients with renal failure with glomerular filtration rates (GFRs) ≤30 ml/min. However, even in patients with normal GFRs, BPs can promote the development of renal failure if administered via the intravenous route without due caution. Overly rapid administration (i.e., over a period of <15 minutes for zoledronate), simultaneous use of potentially nephrotoxic agents (NSAIDs, diuretics), and drug administration to dehydrated patients must be avoided83,84.
Intravenous BPs can result in clinically significant hypocalcaemia, especially when administered to patients with decreased GFRs, vitamin D deficiency, insufficient calcium intake, or very high bone turnover.
The risk of developing osteonecrosis of the jaw (ONJ) among patients treated with BPs for osteoporosis is very low (1/1,500 –1/100,000 patient-years, depending on the specific study)85,86. The incidence of this complication is related to the patient’s state of oral health (i.e., periodontitis) and a history of dental trauma; a decrease in bone turnover is most likely involved. However, BTM measurements are not useful for identifying people at risk. Temporary suspension of drug treatment does reduce the frequency of this complication.
The incidence of atypical fractures of the femur (AFF) is very low87,88. In a recent study from the United States, 1.7 patients with AFF were identified for every 10,000 treated with BPs. The relative risk (RR), compared with those not treated, increased with the time of exposure to BPs (RR = 2.5 with treatments < three years; RR = 8.9 with treatment for three to five years; RR = 19.9 with five to eight years of treatment, and RR = 43.5 for treatments lasting longer than eight years). Despite the observed increase in RR, the absolute risk is very low compared to the risks associated with osteoporotic fractures. Current estimates suggest that for each atypical fracture appearing during the first three years of treatment, ~270 clinically-relevant fragility fractures are prevented, including 70 hip fractures89. Risk factors for AFF include Asian race, low weight, and femoral curvature. The incidence of AFF appears to decline rapidly after drug withdrawal. The usefulness of the synthetic parathyroid hormone, teriparatide, for the treatment of AFF remains controversial.
Various types of inflammatory reactions of the eye have been described in association with the use of BP (e.g., episcleritis, keratitis, and uveitis). These adverse effects are very infrequent but would require discontinuation of treatment90.
Diffuse osteoarticular and muscular pain can develop in patients undergoing BP drug treatment. The discomfort typically disappears after the drug has been withdrawn91.
10. Denosumab
Denosumab is a monoclonal antibody with a powerful antiresorptive effect that translates into a reduction in the risk of fracture. In general, it has shown greater antiresorptive potency and results in a greater increase in BMD than achieved with BPs.
Denosumab therapy results in reductions in the risk of vertebral, non-vertebral, and hip fractures of ~70%, 20%, and 40% respectively92 (Evidence 1b). A post hoc analysis of these data suggests that its efficacy in reducing hip fracture may be greater in subjects older than 75 years of age93 (Evidence 2b). Its beneficial impact on fracture risk appears to be maintained during treatment and persists for at least 10 years94.
In the months following drug withdrawal, an increase in BTMs and a loss of the bone mass gained with subsequent stabilization at baseline values are observed. In some patients, these responses have been associated with multiple vertebral fractures95. Therefore, any interruption of denosumab therapy should be followed by the administration of a BP for six months following the final dose. However, the ideal regimen has not yet been established (see below)18.
Denosumab is generally well tolerated. It is not associated with an increased risk of neoplasms, cardiovascular events, or infections and is safe to use in patients with diabetes96. As with BPs, the risk of AFF and ONJ is very low. In a study performed with patients treated for a prolonged period (up to 10 years), the risk of AFF was determined to be ~1/10,000 patient-years. The risk of developing ONJ was 1/2,000 patient-years94. Furthermore, denosumab can be used safely in patients with GFRs <30 ml/min and even in those on dialysis with no need for dose adjustment. However, hypocalcaemia may develop, especially in patients with advanced renal failure. Close follow-up will be necessary for these patients, together with an adequate supply of calcium and vitamin D.
In conclusion, denosumab therapy can reduce the incidence of vertebral, non-vertebral, and hip fractures. Thus, this agent has a definitive role in the treatment of osteoporosis (Recommendation A).
11. Strontium ranelate
Strontium ranelate reduces the incidence of vertebral and non-vertebral fractures by ~40% and 16%, respectively97. However, the administration of this agent results in an increased incidence of cardiovascular events. It is not currently available for use in Spain or any other European country.
12. Parathyroid hormone (PTH) 1-34 (teriparatide)
Teriparatide is the amino-terminal (1-34) peptide fragment of human parathyroid hormone (PTH) that promotes bone formation. Administration of teriparatide reduces the risk of vertebral fracture by 65% and non-vertebral fracture by 50%98 (Evidence 1a). While teriparatide has not yet been evaluated in trials designed to assess its specific impact on hip fractures, a review of observational studies suggested reductions of ~56%99. A more recent meta-analysis found that teriparatide therapy resulted in no significant reductions in hip fractures48, although another three reviews concluded that it reduced hip fractures between 56% and 65%61,100,101. One study directly compared the effects of the BP, risedronate, and teriparatide in postmenopausal women with severe osteoporosis and vertebral fractures; the teriparatide-treated group experienced fewer vertebral and clinical fractures than the BP-treated group (5.4% versus 12.0% and 4.8% versus 9.8%, respectively)102. Teriparatide is administered as a daily subcutaneous injection for two years. The benefits with respect to BMD that are achieved with this drug decrease progressively after its withdrawal; thus, sequential treatment with an antiresorptive drug is recommended. Teriparatide is generally well tolerated. Several biological and biosimilar drugs have been approved for clinical use because they have met the standard bioequiva-lence requirements established for these drugs.
In conclusion, teriparatide reduces both vertebral and non-vertebral fractures and, although it is not approved for this indication, it probably also reduces the incidence of hip fractures (Recommendation A).
14. Abaloparatide
Abaloparatide is an analog of the 1-34 region of PTH and is a PTHrP (PTH-related peptide). The results of a clinical trial found that administration of abaloparatide reduced the risk of vertebral and non-vertebral fractures by 86% and 43%, respectively, compared to placebo103.
A recent meta-analysis revealed that the use of this drug resulted in 87%, 50%, and 61% reductions in vertebral, non-vertebral, and wrist fractures respectively104. Abaloparatide is approved for use in the US but not in Europe. It is not available in Spain.
15. Romosozumab
Romosozumab is a sclerostin-neutralizing monoclonal antibody. Sclerostin is a small protein pathway which is essential for osteoblastic activity. Various experimental and clinical studies have shown that romosozumab has a dual effect. Administration of romosozumab increases bone formation and also decreases the rate of bone resorption. The latter effect has been associated with the impact of this drug on levels of the osteoclast NKL. Consistent with its dual effect, romosozumab increases the levels of bone formation markers, such as PINP, and decreases the levels of resorption markers, such as CTX. Romosozumab induces notable increases in BMD in both the spine and the hip. The anabolic effects of this drug disappear after 6–12 months of treatment. Therefore, it is typically administered for periods of one year, after which an antiresorptive agent must be used to maintain or increase BMD.
The results of three pivotal trials and several meta-analyses48,105-108 reveal that treatment with romosozumab for 12 months reduces the incidence of vertebral fractures in postmenopausal women and men with osteoporosis (relative risk reduction [RRR], 66%–73%)109. Likewise, the combined analysis revealed that romosozumab therapy decreases the risk of non-vertebral (RRR 33%) and hip fractures (RRR 56%). In postmenopausal women with severe osteoporosis and a history of previous fragility fractures, treatment with romosozumab for one year followed by alendronate significantly reduced the risk of new vertebral, hip, and clinical fractures, compared with treatment over the entire period with alendronate alone107.
Romosozumab is generally well-tolerated, although the results of several studies suggested that it may increase the incidence of cardiovascular events110. While this difference was small in absolute terms (1.3% of events versus 0.9% in the control group), romosozumab is not indicated in patients with a history of myocardial infarction or stroke and should be carefully considered in patients presenting with multiple cardiovascular risk factors.
In conclusion, romosozumab has a defined role in the treatment of osteoporosis as it reduces the risk of both vertebral and peripheral fractures (Recommendation A). Potential cardiovascular risks and specific contraindications should be assessed in each patient.
16. Vertebroplasty and kyphoplasty
Although many uncontrolled studies have shown that these procedures are associated with a marked analgesic effect, randomised clinical trials have offered contradictory re-sults111-114 and controversy regarding a potential increased risk of fracture in the adjacent vertebrae remains. Therefore, these procedures are not routinely recommended87 for patients with asymptomatic vertebral fractures, mild pain, or those with symptoms that have persisted for more than one year. These procedures can be considered in patients who present with fractures that are less than six weeks old and severe pain despite appropriate medical treatment and in patients with fractures that have evolved over six weeks to one year ago with persistent pain that responds poorly to analgesics and evidence of edema on magnetic resonance imaging studies115. These procedures may also be useful in patients who present with contraindications or poor tolerance to analgesics. Vertebroplasty and kyphoplasty are similar in terms of effectiveness and safety116. There is insufficient evidence on the relative usefulness of procedures that include the insertion of expanding implants specifically when compared to vertebroplasty and balloon kyphoplasty (Recommendation B).
In conclusion, vertebroplasty, kyphoplasty, and related techniques are not routinely recommended for the treatment of vertebral fractures, although these procedures may help to control symptoms in carefully selected patients (Recommendation C). In any case, its use must be accompanied by medical treatment of osteoporosis to prevent new fractures.
INITIATION AND FOLLOW-UP OF TREATMENT
1. Decision to commence treatment
There is no internationally agreed or apstandard on when to initiate treatment for osteoporosis. SEIOMM suggests that, in general, patients that present with the following attributes should be treated:
Patients who present with one or more fragility fractures, especially those of the vertebrae, hip, humerus, and pelvis, regardless of whether their T-scores indicate "osteoporosis".
Patients with a BMD <-2.5T at the lumbar spine, femoral neck, or total hip.
Women with osteopenia (particularly with T < -2.0) who also present with factors that are strongly-associated with an increased risk of fracture (e. g., hypogonadism or early menopause, treatment with glucocorticoids or antiestrogens, among others).
However, we recognise that some situations may require exceptions to these recommendations. All patients must undergo a careful, individualised assessment that considers the risk factors for fracture as well as other clinical characteristics. For example, it may be possible to delay the start of treatment in young women who present with only slightly low BMD, without fractures or other risk factors. By contrast, a patient who presents with several important risk factors may require early treatment. Scales that help estimate fracture risk (e. g., FRAX) may be helpful, although these instruments have not yet been fully validated for use in the Spanish population, as mentioned above.
2. Control of the therapeutic response
Adherence and therapeutic responses to treatment regimens can be assessed by changes in BTMs.
The beneficial effect of a given treatment regimen can be confirmed by increases in BMD and the absence of new fractures. However, it is critical to recognise that a single fracture while on a treatment regimen is not necessarily indicative of therapeutic failure. Elderly patients and those with dementia, poor quality of life, and/or multiple fractures are at greater risk for therapeutic failure. In cases where oral BPs have failed, parenteral drugs (zoledronate, denosumab, and [depending on patient characteristics] teriparatide or romosozumab) may represent good therapeutic alternatives.
Changes to treatment regimens due to a potential inadequate response may be considered in the following circumstances117:
development of two successive fractures; or
coincidence of two of the following three factors, including the de velopment of a new fracture; decrease in BMD greater than the minimum significant change (nb: this varies based on the densitometer and the skeletal region studied but is usually between 4–5%); or decrease in BTMs below the minimum significant change, usually ~25% (Recommendation D).
Before proceeding with a therapeutic change, the following factors should be considered as possible causes of an inadequate response: a) vitamin D deficiency; b) secondary forms of osteoporosis; c) inadequate compliance; d) tendency to fall; e) defective techniques used to measure BMD and/or BTMs; f) serious bone deterioration, leading to the likelihood of new fractures despite active drug treatment.
If the reasons for the changes observed include an apparent lack of an appropriate response, the following options are recommended117,118 (Recommendation D):
3. Duration of treatment
Interruption of treatment is justified when the risk/benefit ratio becomes unfavourable. These situations can include: a) therapeutic objectives have been achieved;
b) loss of effectiveness; or c) increased risk of developing secondary effects.
-
Attainment of objectives
Although the "treat to target" strategy is theoretically an attractive approach, the objectives to be achieved in the treatment of osteoporosis are not well defined, which limits its practical application. For some experts, the absence of new fractures and the increase in BMD would be the most appropriate objectives to consider. Other experts have recommended objectives that include reaching a T-score greater than -2.0 or -2.5, especially in studies focused on the hip119-121.
-
Loss of effectiveness
The increase in BMD induced by antiresorptive drugs is more marked during the first years of treatment. However, that does not mean that these drugs subsequently lose effectiveness. Although there is no general agreement, the results of several studies have revealed that fracture risk reduction persists with treatment with zoledronate for six years and with alendronate or denosumab for 10 years, especially in patients who maintain a high baseline risk.
-
Increased risk of developing undesirable long-term side effects
ONJ and AFF induced by BPs and denosumab are particularly relevant to this concern. The absolute risk of ONJ in patients treated with antiresorptive agents for osteoporosis is very low and similar to that reported for the general population. Likewise, there is currently no evidence that short-term discontinuation of treatment reduces the risk of ONJ or disease progression in patients who need dental procedures. The absolute risk of AFF is also very low, although the relative risk increases with the duration of exposure to BPs (see the previous section).
Based on these facts, the following recommendations are proposed. These recommendations represent expert consensus, albeit without published studies to provide definitive support122-126 (Recommendation D):
1. Patients treated with BPs should be evaluated after three (zoledronate) or five years (oral BP) of treatment. Patients treated with denosumab should be evaluated after 5–10 years of treatment.
-
2. After this evaluation, treatment should be continued (with the same or another drug) if any of the following circumstances occur:
If none of these circumstances arise, BP treatment can be withdrawn, at least temporarily.
If treatment is maintained, the possibility of its withdrawal should be periodically reassessed at various intervals thereafter. There is currently no guidance as to how often each patient should be reassessed, nor if there is a defined maximum duration of treatment. A limit of 10 years is often set, as there are no studies that have evaluated the impact of these drugs over the longer term. However, if the patient remains at risk, anti-osteoporotic treatment should not be withdrawn. If anti-resorptive treatment is withdrawn, and the patient remains at risk for fracture, a drug from another class should be administered, for example, an anabolic.
When BP treatment is withdrawn, the suspension must be temporary (i.e., a “drug holiday”). It is not known how long the treatment regimen can be safely suspended or fully discontinued. Typically, the drug can be suspended for a period of 1 to 3 years, depending on the BP used (e. g., perhaps one year for risedronate, two years for alendronate, and three years for zoledronate). Some experts have suggested that BTMs and BMD measurements can help with this decision, although we are not in a position to confirm this. In theory, if the BMD remains above a “target” value (e. g., T >-2 or -2.5), drug withdrawal can be considered.
“Drug holidays” should not be scheduled for patients treated with denosumab, because after its withdrawal, not only is there no residual effect, but bone turnover increases to levels above baseline values (i. e., a “rebound effect”). This increased bone turnover has been associated with a rapid loss of bone mass and an increased risk of developing multiple vertebral fractures. Therefore, continuing denosumab therapy indefinitely is recommended. In cases in which denosumab must be discontinued, it should be replaced with a potent BP (see below)18.
There are some data available that address the efficacy and safety of SERMs (raloxifene and bazedoxifene) for up to eight years. In these cases, the treatment regimen can be maintained through this time or until the risk of hip fracture or complications, such as thromboembolic disease, increases. It is not usually recommended in patients older than 65-70 years of age.
Treatment with teriparatide or romosozumab should be maintained for 24 and 12 months, respectively, followed in both cases by an antiresorptive drug.
4. Sequential and combined treatment
4.1. Bisphosphonates (BPs) after denosumab
As stated above, a BP must be administered after discontinuation of denosumab to limit the rebound effect (Recommendation A). Pending the results of ongoing trials focused on the optimal BP regimen, patients with a low risk of fracture and who have been treated with denosumab for a relatively short period (up to 2.5 years), can be treated for another two years with an oral BP, such as alendronate. IV zoledronate is another alternative. Zoledronate is preferable in cases of prior intolerance to oral BPs, foreseeable poor adherence, or polypharmacy. Patients who have been treated with denosumab for a longer period (i. e., more than 2.5 years) or who remain at high risk of fracture should be treated with zoledronate for 1–2 years. The first dose of zoledronate should be administered once denosumab has been discontinued (i. e., six months after the last dose) and repeated when elevations in BTMs are detected, generally at 6 or 12 months later. If BTM measurements are not available, zoledronate administration can be repeated 6 and 12 months after the first dose18,127. The need for additional doses should be considered on an individual basis (Recommendation D).
There are no trials that established the best therapeutic options for patients who have sustained a vertebral fracture after discontinuation of denosumab. However, the following options have been recommended in this situation:
(Recommendation D)18. In the months following the discontinuation of denosumab, treatment with teriparatide alone should be avoided, because it causes a transient loss of bone mass128.
4.2. Antiresorptive agents after anabolics
Progressive loss of BMD will follow after discontinuing treatment with teriparatide129. Several studies have shown that this loss of bone mass could be prevented by the sequential administration of an antiresorptive agent; additional increases in BMD might also result from this new drug regimen130, although there are no data available on fracture prevention. Likewise, after completion of treatment with romosozumab, current recommendations include that the patient shouldontinue with an antiresorptive agent131,132.
In conclusion, after completion of treatment with anabolic drugs, such as teriparatide or romosozumab, further treatment with powerful antiresorptive drugs, such as a BP or denosumab, is recommended (Recommendation A).
4.3. Anabolic after antiresorptive drugs
The anabolic effects of PTH depend on the type of antiresorptive drug used in the previous treatment regimen. Several studies have confirmed that the previous use of a BP result in an overall decrease and slightly reduces the rate of increase in BMD that resulted from teriparatide treatment133,134. However, the reduction in fracture risk associated with the use of teriparatide is not affected by prior treatment with a BP135.
One study focused on the impact of switching to romosozumab or teriparatide among women previously treated with a BP (particularly alendronate). Both groups exhibited increases in spine BMD, but those who switched to romosozumab exhibited these increases 12 months or more after those achieved in patients who switched to teriparatide; this was especially notable in the hip136.
By contrast, initiation of teriparatide in postmenopausal women who had completed a course of treatment with denosumab resulted in a transient decrease in BMD128. Therefore, teriparatide should not be administered after discontinuation of denosumab.
In conclusion, although the preferred sequence is an anabolic followed by an antiresorptive drug, prior treatment with a BP is not a contraindication for subsequent administration of teriparatide or romosozumab and is considered adequate to reduce the risk of fracture. (Recommendation A). Teriparatide in the months following denosumab suspension should be avoided, given the risk of accelerated bone loss (Recommendation A).
4.4. Combination treatments
The combination of two antiresorptive drugs (e.g., estrogens and a BP) can enhance the gain in bone mass achieved individually137, but there are doubts regarding the risk-benefit ratio of this association compared to results achieved with each drug alone. This combination is not recommended.
Studies focused on the combination of a BP and teriparatide have not shown clear benefits over individual administration of each drug. Thus, this combination is not recommended. However, in one study, the combination of zoledronate and teriparatide resulted in a higher value for hip BMD than what was achieved in response to teriparatide alone138.
In one trial, the use of denosumab combined with teriparatide resulted in greater increases in BMD at the hip and spine than those achieved with each drug alone139.
In conclusion, given the lack of data on fracture prevention and the higher costs and side effects associated with these types of regimens, combination therapy is not generally recommended at this time. However, combinations of denosumab or zoledronate with teriparatide can be considered on an individual basis in particularly severe cases associated with a very high risk of hip fracture. In these cases, it may be preferable to delay the start of antiresorptive for one to two months after initiating teriparatide to take advantage of the anabolic effect (Recommendation grade D).
5. Therapeutic decision algorithms
The proposed algorithm is based on data from published trials and considerations that are summarised below.
5.1. Initial treatment (Choice of a drug; Figure 1)
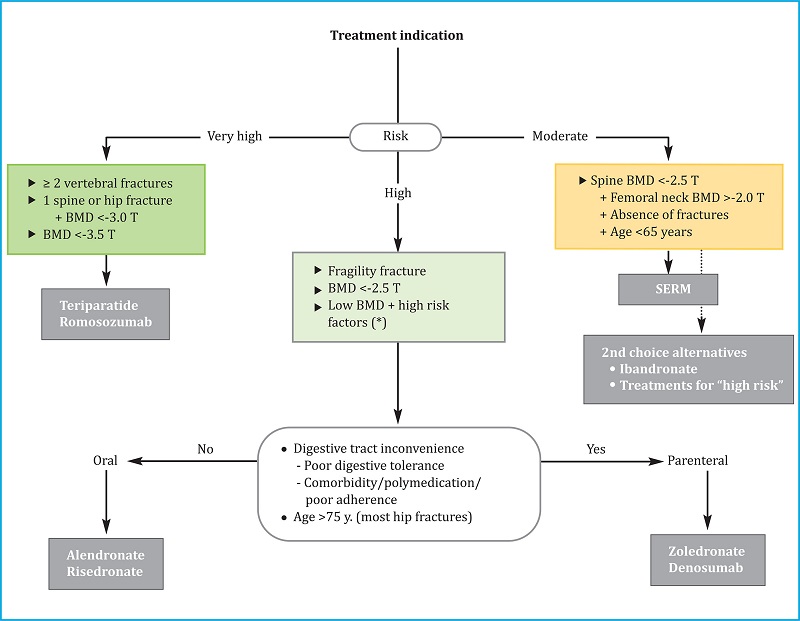
SERM: selective estrogen receptor modulator; (*): especially if T ≤-2 and factors strongly associated with fracture risk, such as hypogonadism, early menopause, or treatment with glucocorticoids or sex hormone antagonists. These general criteria may need to be adapted based on other clinical determinants of fracture risk, the characteristics of individual patients, and their preferences.
Figure 1. Algorithm for selecting the initial treatment in postmenopausal osteoporosis
The main criterion for choosing the initial drug is the risk of fracture. We distinguish three levels of risk, including “moderate”, “high”, and “very high”.
1) Moderate risk. This category corresponds to the risk profile of a woman under 65 years of age, with no history of fracture, a spinal T-score between -2.5 and -3.0, and a relatively preserved hip BMD (T-score >-2). In this situation, a SERM is recommended because one can then delay the use of prolonged treatment strategies that can elicit AFF or ONJ. However, ibandronate and antiresorptive agents that are typically recommended for high-risk situations are the second choice in this situation. These drugs represent acceptable alternatives if for some reason SERMs are to be avoided.
2) High risk. Most of the cases seen in the clinic will present this level of risk (see section above “Decision to start treatment”). These patients do not meet the criteria that define either moderate or very high-risk cohorts as described further below. Alendronate, risedronate, zoledronate, or denosumab are indicated for the treatment of patients in the high-risk cohort. Oral BPs are considered preferable for patients <75 years of age when there are no inconveniences with respect to oral administration (digestive problems, polypharmacy, adherence). Injectable antiresorptive drugs are considered preferable in all other cases. As most individuals who have sustained hip fractures are over 75 years of age and belong to the second group, injectable antiresorptive agents are generally preferred for this group. Given the rebound effect after discontinuation of denosumab, zoledronate may be the preferred agent if there are doubts regarding compliance.
3) Very high risk. We consider women to be at very high risk in any of the following situations: a) two or more vertebral fractures, or an equivalent risk (i.e., T-score <-3.5); or b) vertebral or hip fracture with a T-score <-3.0. There may be other clinical situations that suggest that a patient is at a very high risk of fracture; these will require individualised consideration. For this level of risk, bone-forming drugs such as teriparatide or romosozumab should be used. Romosozumab may have a better cost-benefit ratio (although its marketing price was not known at the time that these guidelines were written), albeit a less favorable risk-benefit ratio due to the potential increase in cardiovascular events. Romosozumab should be avoided in all patients with or at high risk of developing cardiovascular disease. However, these guidelines and recommendations should be understood as provisional at this time, pending marketing in Spain and further experience with this drug in our population.
Although some authors have suggested that all patients with a recent fracture, especially a vertebral fracture, might benefit from treatment with a bone-forming drug. However, there is currently no consensus on this point among our panel of experts. Regardless of the treatment that is ultimately selected, therapy should be initiated as soon as possible given that these patients are at very high risk for new fractures.
5.2. Long-term treatment (Figure 2)
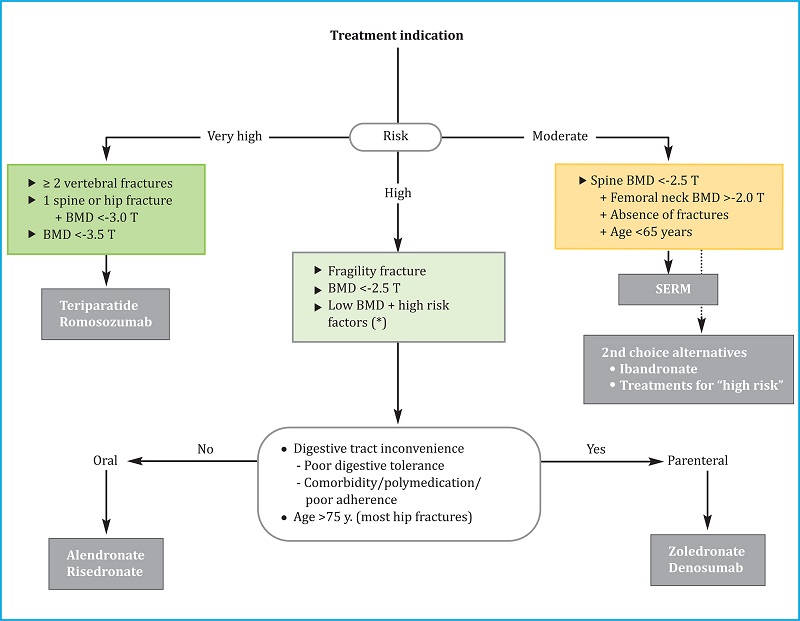
BP: bisphosphonates; SERM: selective estrogen receptor modulators; BTM: bone turnover markers; (*): there are not enough data to establish a recommendation after that treatment time, so the possible options are listed before a decision that must be individualized.
Figure 2. Long-term treatment continuation algorithm
Romosozumab should only be administered for one year; teriparatide therapy is limited to two years. Likewise, given that efficacy and safety data are available for up to eight years of treatment only, withdrawal of SERMs should be considered after that period, when the patient reaches 65-70 years of age or if the risk of fracture increases. After one or more of these milestones are reached, it will likely be necessary to administer another antiresorptive. The discussion on long-term treatment is thus restricted to a consideration of BPs and denosumab. One key differentiating factor at this time is the potential impact of a temporary interruption or “therapeutic vacation” or “drug holiday”. While this is discouraged for individuals undergoing treatment with denosumab, it is currently accepted for BP regimens.
1) Denosumab. This agent can be administered continuously for 5–10 years. No information is currently available regarding longer periods of use. Thus, the decision to continue or discontinue drug treatment should be made carefully. Once administration of denosumab has been interrupted, the patient should be treated with a BP, for example, alendronate or zoledronate. Zoledronate is preferred if denosumab treatment was prolonged for more than 2–3 years. (See section 4.1).
2) Bisphosphonates (BPs). Three periods of use have been described:
First period: Current recommendations suggest that these drugs should be administered without interruption for five years (for oral BPs) or three years for zoledronate.
Second period: After the first period (above), treatment can be temporarily interrupted if the requirements for a "drug holiday" are met (see above). The need to reinstate treatment should be periodically assessed. Once reinstated, the possibility of a second temporary suspension can be reassessed at frequent intervals.
-
Third period (after 10 years of continuous or intermittent treatment with an oral BP, or six years of treatment with zoledronate): No high-quality studies are available that can be used to guide decision-making. By extrapolation of what was proposed for the second period, it is reasonable to assume that a patient that meets the appropriate requirements can be converted to a "drug holiday" regimen. Otherwise, one of the following three options should be chosen depending on context and clinical judgement:
Maintain treatment: This increases the risk of complications but may keep the risk of osteoporotic fractures comparatively low;
Withdraw treatment: This strategy reduces the risk of complications but could increase the risk of developing osteoporotic fractures;
Change the regimen: Teriparatide can be prescribed. This drug can reduce the risk of complications as well as the risk of developing osteoporotic fractures.
MALE OSTEOPOROSIS
There is very little evidence available to guide the treatment of male osteoporosis. Of the information that does exist, most of the studies focus on increasing BMD as a primary objective. The results are largely similar to those obtained from studies in women and suggest that drug efficacy in men is similar with respect to the prevention of fractures. Interestingly, administration of BPs such as alendronate, risedronate, and zoledronate resulted in a decrease in vertebral fractures in male patients140-144. Denosumab reportedly increases BMD in men and reduces the risk of fracture specifically in those undergoing androgen deprivation therapy145,146. Teriparatide also has beneficial effects in men147,148. For this reason, a drug selection strategy similar to that designed initially for women might be proposed for men:
Risedronate or alendronate (nb: the latter drug is not approved in Spain for male osteoporosis) for patients who have no restrictive criteria for oral administration, as described for women with postmenopausal osteoporosis;
Zoledronate or denosumab in patients with these restrictive criteria or who are older and therefore are at a higher risk of hip fracture;
Teriparatide in patients with established osteoporosis and with a high risk of fracture. Although, as in women, romosozumab also induces gains in BMD in men109, its use to treat osteoporosis in men is not currently approved.
Proper calcium intake is also recommended, preferably through diet and vitamin D supplements in cases of insufficiency. Androgens are only justified if there is associated hypogonadism and no contraindications for their use. Even in cases of hypogonadism, some of the aforementioned drugs might have significant anti-fracture efficacy. Lastly, when hypercalciuria is detected, administration of thiazides may be considered (Recommendation D).
GLUCOCORTICOID-INDUCED OSTEOPOROSIS
BPs are the drugs of choice for glucocorticoid-induced osteoporosis149-151. However, if a patient presents with vertebral fractures, treatment with teriparatide is justified due to its greater anti-fracture effect152,153 (Recommendation A). Calcium and vitamin D should also be administered. The active metabolites of vitamin D by themselves have some preventive effect on bone loss, but we do not have convincing evidence regarding their role in fracture prevention at this time154.
Postmenopausal women and men over the age of 50 years who receive or are about to receive doses of prednisone equal to or greater than 5 mg/day (or the equivalent dose of other corticosteroids) for more than three months should receive treatment for this condition. In premenopausal women and men under 50 years of age, treatment is indicated only in cases of previous fractures, low BMD, or very high glucocorticoids dose (e. g., >30 mg/day of prednisone for more than 3 months). Drug treatment should be maintained while the patient remains on corticosteroids. Once they are withdrawn, the risk of fracture must be evaluated in each patient. If the risk is not overly high, it may be possible to stop osteoporosis therapy entirely.
Denosumab results in a greater increase in BMD than that achieved by BPs in patients receiving corticosteroids. However, the reduction in fracture risk is similar with both drugs, as are the adverse effects155-157. Given, on the one hand, the rebound effect observed in some patients when denosumab is discontinued158 and, likewise, the possibility of withdrawing antiresorptive treatment when discontinuing corticosteroids, denosumab should be indicated when it is not possible to use other antiresorptive agents and the risk of fracture is high.
In patients receiving corticosteroids, densitometric evaluation performed at shorter intervals may be justified (Recommendation D).
We thank Monica Silvan for her administrative assistance.
REFERENCES
1 González-Macías J, Del Pino-Montes J, Olmos JM, Nogués X. Guías de práctica clínica en la osteoporosis posmenopáusica, glucocorticoidea y del varón. Sociedad Española de Investigación Ósea y del Metabolismo Mineral (3.a versión actualizada 2014). Rev Clín Esp. 2015;215(9):515-526. [ Links ]
2 US Department of Health and Human Services. Bone health and osteoporosis: a report of the Surgeon General. US Heal Hum Serv. 2004;437. [ Links ]
3 Ambrose AF, Paul G, Hausdorff JM. Risk factors for falls among older adults: A review of the literature. Maturitas. 2013;75(1):51-61. [ Links ]
4 Roux C, Briot K. Imminent fracture risk. Osteoporos Int. 2017;28:1765-1769. [ Links ]
5 Balasubramanian A, Zhang J, Chen L, Wenkert D, Daigle S, Grauer A, et al. Risk of subsequent fracture after prior fracture among older women. Osteoporos Int. 2019;30:79-92. [ Links ]
6 Hannan M, Weycker D, McLean R, Sahni S, Bornheimer R, Barron R, et al. Predictors of Imminent Risk of Nonvertebral Fracture in Older, High-Risk Women: The Framingham Osteoporosis Study. JBMR plus. 2019;3:e10129. [ Links ]
7 Marshall D, Johnell O, Wedel H. Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. Br Med J. 1996; 312(7041):1254-1259. [ Links ]
8 Kanis JA, Melton LJ, Christiansen C, Johnston CC, Khaltaev N. The diagnosis of osteoporosis. J Bone Miner Res. 1994;9(8):1137-1141. [ Links ]
9 Kanis JA, McCloskey EV, Johansson H, Oden A, Melton LJ, Khaltaev N. A reference standard for the description of osteoporosis. Bone. 2008;42(3):467-475. [ Links ]
10 Lewiecki EM, Gordon CM, Baim S, Leonard MB, Bishop NJ, Bianchi M-L, et al. International Society for Clinical Densitometry 2007 Adult and Pediatric Official Positions. Bone. 2008;43 (6):1115-1121. [ Links ]
11 Schousboe JT, Shepherd JA, Bilezikian JP, Baim S. Executive Summary of the 2013 International Society for Clinical Densitometry Position Development Conference on Bone Densitometry. J Clin Densitom. 2013;16(4):455-466. [ Links ]
12 Sornay-Rendu E, Munoz F, Garnero P, Duboeuf F, Delmas PD. Identification of osteopenic women at high risk of fracture: the OFELY study. J Bone Miner Res. 2005;20:1813-1819. [ Links ]
13 Schuit SCE, Van Der Klift M, Weel AEAM, De Laet CEDH, Burger H, Seeman E, et al. Fracture incidence and association with bone mineral density in elderly men and women: The Rotterdam Study. Bone. 2004;34(1):195-202. [ Links ]
14 Nelson HD, Helfand M, Woolf SH, Allan JD. Screening for Postmenopausal Osteoporosis: A Review of the Evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2002;137(6):529. [ Links ]
15 Kanis JA, Oden A, Johnell O, Johansson H, De Laet C, Brown J, et al. The use of clinical risk factors enhances the performance of BMD in the prediction of hip and osteoporotic fractures in men and women. Osteoporos Int. 2007;18 (8):1033-1046. [ Links ]
16 Hochberg MC, Greenspan S, Wasnich RD, Miller P, Thompson DE, Ross PD. Changes in bone density and turnover explain the reductions in incidence of nonvertebral fractures that occur during treatment with antiresorptive agents. J Clin Endocrinol Metab. 2002; 87:1586-1592. [ Links ]
17 Eastell R, Szulc P. Use of bone turnover markers in postmenopausal osteoporosis. Lancet Diabetes Endocrinol. 2017;5(11):908-923. [ Links ]
18 Tsourdi E, Zillikens MC, Meier C, Body J-J, Gonzalez Rodriguez E, Anastasilakis AD, et al. Fracture Risk and Management of Discontinuation of Denosumab Therapy: A Systematic Review and Position Statement by ECTS. J Clin Endocrinol Metab. 2020; 106(1):264-281. [ Links ]
19 Seeley DG, Browner WS, Nevitt MC, Genant HK, Dcott JC, Cummings SR. Which fractures are associated with low apendicular bone mass. Ann Intern Med. 1991;115:837-842. [ Links ]
20 Jiang G, Eastell R, Barrington NA, Ferrar L. Comparison of methods for the visual identification of prevalent vertebral fracture in osteoporosis. Osteoporos Int. 2004;15(11):887-896. [ Links ]
21 Schousboe JT, Vokes T, Broy SB, Ferrar L, McKiernan F, Roux C, et al. Vertebral Fracture Assessment: The 2007 ISCD Official Positions. J Clin Densitom. 2008;11(1):92-108. [ Links ]
22 Koh LKH, Ben Sedrine W, Torralba TP, Kung A, Fujiwara S, Chan SP, et al. A Simple Tool to Identify Asian Women at Increased Risk of Osteoporosis. Osteoporos Int. 2001;12(8):699-705. [ Links ]
23 Richy F, Gourlay M, Ross PD, Sen SS, Radican L, De Ceulaer F, et al. Validation and comparative evaluation of the osteoporosis self-assessment tool (OST) in a Caucasian population from Belgium. QJM. 2004;97(1):39-46. [ Links ]
24 Kanis JA, Johnell O, Oden A, Johansson H, McCloskey E. FRAX and the assessment of fracture probability in men and women from the UK. Osteoporos Int. 2008;19(4):385-397. [ Links ]
25 Nguyen ND, Frost SA, Center JR, Eisman JA, Nguyen TV. Development of prognostic nomograms for individualizing 5-year and 10-year fracture risks. Osteoporos Int. 2008;19(10): 1431-1444. [ Links ]
26 Hippisley-Cox J, Coupland C. Predicting risk of osteoporotic fracture in men and women in England and Wales: prospective derivation and validation of QFractureScores. BMJ. 2009; 339:b4229–b4229. [ Links ]
27 Bolland MJ, Siu ATY, Mason BH, Horne AM, Ames RW, Grey AB, et al. Evaluation of the FRAX and Garvan fracture risk calculators in older women. J Bone Miner Res. 2011;26(2):420-427. [ Links ]
28 Cummins NM, Poku EK, Towler MR, O’Driscoll OM, Ralston SH. Clinical Risk Factors for Osteoporosis in Ireland and the UK: A Comparison of FRAX and QFractureScores. Calcif Tissue Int. 2011;89(2):172-177. [ Links ]
29 González-Macías J, Marin F, Vila J, Díez-Pérez A. Probability of fractures predicted by FRAX® and observed incidence in the Spanish ECOSAP Study cohort. Bone. 2012;50(1):373-377. [ Links ]
30 Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96(1):53-58. [ Links ]
31 Goodwin VA, Abbott RA, Whear R, Bethel A, Ukoumunne OC, Thompson-Coon J, et al. Multiple component interventions for preventing falls and fall-related injuries among older people: systematic review and meta-analysis. BMC Geriatr. 2014;14:15. [ Links ]
32 Kanis JA, Johnell O, Oden A, Johansson H, De Laet C, Eisman JA, et al. Smoking and fracture risk: a meta-analysis. Osteoporos Int. 2005;16(2):155-162. [ Links ]
33 Kanis JA, Johansson H, Johnell O, Oden A, De Laet C, Eisman JA, et al. Alcohol intake as a risk factor for fracture. Osteoporos Int. 2005;16:737-742. [ Links ]
34 Morello RT, Soh S-E, Behm K, Egan A, Ayton D, Hill K, et al. Multifactorial falls prevention programmes for older adults presenting to the emergency department with a fall: systematic review and meta-analysis. Inj Prev. 2019; 25(6):557-564. [ Links ]
35 Vlaeyen E, Coussement J, Leysens G, Van der Elst E, Delbaere K, Cambier D, et al. Characteristics and Effectiveness of Fall Prevention Programs in Nur-sing Homes: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J Am Geriatr Soc. 2015;63 (2):211-221. [ Links ]
36 Santesso N, Carrasco-Labra A, Brignardello-Petersen R. Hip protectors for preventing hip fractures in older people. Cochrane Database Syst Rev. 2014; [ Links ]
37 Peris P, Martínez-Ferrer A, Monegal A, Martínez de Osaba MJ, Muxi A, Guañabens N. 25 hydroxyvitamin D serum levels influence adequate response to bisphosphonate treatment in postmenopausal osteoporosis. Bone. 2012;51 (1):54-58. [ Links ]
38 Olmos JM, Hernández JL, Llorca J, Nan D, Valero C, González-Macías J. Effects of 25-Hydroxyvitamin D3 Therapy on Bone Turnover Markers and PTH Levels in Postmenopausal Osteoporotic Women Treated with Alendronate. J Clin Endocrinol Metab. 2012;97(12): 4491-4497. [ Links ]
39 Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Evaluation, Treatment, and Prevention of Vitamin D Deficiency: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2011;96(7):1911-1930. [ Links ]
40 Sanders KM, Stuart AL, Williamson EJ, Simpson JA, Kotowicz MA, Young D, et al. Annual High-Dose Oral Vitamin D and Falls and Fractures in Older Women. JAMA. 2010;303(18):1815. [ Links ]
41 Eleni A, Panagiotis P. A systematic review and metaanalysis of vitamin D and calcium in preventing osteoporotic fractures. Clin Rheumatol. 2020; 39:3571-3579. [ Links ]
42 Yao P, Bennett D, Mafham M, Lin X, Chen Z, Armitage J, et al. Vitamin D and Calcium for the Prevention of Fracture: A Systematic Review and Meta-analysis. Vol. 2, JAMA network open. 2019;2: e1917789. [ Links ]
43 Zhao JG, Zeng XT, Wang J, Liu L. Association between calcium or Vitamin D supplementation and fracture incidence in community-dwelling older adults a systematic review and meta-analysis. JAMA. 2017;318:466-482. [ Links ]
44 Overman RA, Borse M, Gourlay ML. Salmon Calcitonin Use and Associated Cancer Risk. Ann Pharmacother. 2013;47(12):1675–1684. [ Links ]
45 Knopp-Sihota JA, Newburn-Cook C V, Homik J, Cummings GG, Voaklander D. Calcitonin for treating acute and chronic pain of recent and remote osteoporotic vertebral compression fractures: a systematic review and meta-analysis. Osteoporos Int. 2011;23(1):17-38. [ Links ]
46 Xiao X, Xu Y, Wu Q. Thiazide diuretic usage and risk of fracture: a meta-analysis of cohort studies. Osteoporos Int. 2018;29(7):1515-1524. [ Links ]
47 Cheng L, Zhang K, Zhang Z. Effectiveness of thiazides on serum and urinary calcium levels and bone mineral density in patients with osteoporosis: a systematic review and meta-analysis. Drug Des Devel Ther. 2018;12: 3929-3935. [ Links ]
48 Barrionuevo P, Kapoor E, Asi N, Alahdab F, Mohammed K, Benkhadra K, et al. Efficacy of pharmacological therapies for the prevention of fractures in postmenopausal women: A network meta-analysis. J Clin Endocrinol Metab. 2019;104(5):1623-1630. [ Links ]
49 Anderson GL, Limacher M, Assaf AR, Bassford T, Beresford SA, Black H, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. J Am Med Assoc. 2004;291:1701-1712. [ Links ]
50 Siris ES, Harris ST, Eastell R, Zanchetta JR, Goemaere S, Diez-Perez A, et al. Skeletal effects of raloxifene after 8 years: Results from the Continuing Outcomes Relevant to Evista (CORE) study. J Bone Miner Res. 2005; 20(9): 1514-1524. [ Links ]
51 Ettinger B, Black DM, Mitlak BH, Knickerbocker RK, Nickelsen T, Genant HK. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene. Results from a 3-year randomized clinical trial. J Am Med Assoc. 1999; 282:637-645. [ Links ]
52 Cummings SR, Ettinger B, Delmas PD, Kenemans P, Stathopoulos V, Verweij P, et al. The effects of tibolone in older postmenopausal women. N Engl J Med. 2008;359(7):697-708. [ Links ]
53 Formoso G, Perrone E, Maltoni S, Balduzzi S, Wilkinson J, Basevi V, et al. Short-term and long-term effects of tibolone in postmenopausal women. Cochrane Database of Systematic Reviews. 2016;10:CD008536. [ Links ]
54 Lambert MNT, Hu LM, Jeppesen PB. A systematic review and meta-analysis of the effects of isoflavone formulations against estrogen-deficient bone resorption in peri-and-postmenopausal women. Am J Clin Nutr. 2017;106: 801-811. [ Links ]
55 Wells GA, Cranney A, Peterson J, Boucher M, Shea B, Robinson V, et al. Etidronate for the primary and secondary prevention of osteoporotic fractures in postmenopausal women. Cochrane database Syst Rev. 2008 23:CD003376. [ Links ]
56 Black DM, Thompson DE, Bauer DC, Ensrud K, Musliner T, Hochberg MC, et al. Fracture risk reduction with alendronate in women with osteoporosis: the fracture intervention trial. J Clin Endocrinol Metab. 2000;85: 4118-4124. [ Links ]
57 Wells GA, Cranney A, Peterson J, Boucher M, Shea B, Welch V, et al. Alendronate for the primary and secondary prevention of osteoporotic fractures in postmenopausal women. Cochrane Database Syst Rev. 2008;23:CD001155. [ Links ]
58 Bauer DC, Schwartz A, Palermo L, Cauley J, Hochberg M, Santora A, et al. Fracture prediction after discontinuation of 4 to 5 years of alendronate therapy: the FLEX study. JAMA Intern Med. 2014;174(7):1126-1134. [ Links ]
59 Black DM, Schwartz A V, Ensrud KE, Cauley JA, Levis S, Quandt SA, et al. Effects of Continuing or Stopping Alendronate After 5 Years of Treatment. JAMA. 2006;296(24):2927. [ Links ]
60 Schwartz AV, Bauer DC, Cummings SR, Cauley JA, Ensrud KE, Palermo L, et al. Efficacy of continued alendronate for fractures in women with and without prevalent vertebral fracture: The FLEX Trial. J Bone Miner Res. 2010;25(5): 976-982. [ Links ]
61 Ding L-L, Wen F, Wang H, Wang D-H, Liu Q, Mo Y-X, et al. Osteoporosis drugs for prevention of clinical fracture in white postmenopausal women: a network meta-analysis of survival data. Osteoporos Int. 2020;31(5):961-971. [ Links ]
62 Sing C-W, Wong AYS, Kiel DP, Cheung EYN, Lam JKY, Cheung TT, et al. Association of Alendronate and Risk of Cardiovascular Events in Patients With Hip Fracture. J Bone Miner Res. 2018; 33(8):1422-1434. [ Links ]
63 Harris ST, Watts NB, Genant HK, McKeever CD, Hangartner T, Kelelr M, et al. Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis. A randomized controlled trial. J Am Med Assoc. 1999;282:1344-1352. [ Links ]
64 McClung MR, Balske A, Burgio DE, Wenderoth D, Recker RR. Treatment of postmenopausal osteoporosis with delayed-release risedronate 35 mg weekly for 2 years. Osteoporos Int. 2013;24(1):301-310. [ Links ]
65 Delmas PD, Benhamou CL, Man Z, Tlustochowicz W, Matzkin E, Eusebio R, et al. Monthly dosing of 75 mg risedronate on 2 consecutive days a month: efficacy and safety results. Osteoporos Int. 2007;19(7):1039-1045. [ Links ]
66 Black DM, Delmas PD, Eastell R, Reid IR, Boonen S, Cauley JA, et al. Once-Yearly Zoledronic Acid for Treatment of Postmenopausal Osteoporosis. N Engl J Med. 2007;356(18):1809-1822. [ Links ]
67 Black DM, Reid IR, Cauley J, Boonen S, Cosman F, Leung P, et al. The effect of 3 versus 6 years of zoledronic acid treatment in osteoporosis: A randomized extension to the HORIZON-Pivotal Fracture Trial (PFT). Bone. 2011;48: 1298-1304. [ Links ]
68 Reid IR, Horne AM, Mihov B, Stewart A, Garratt E, Wong S, et al. Fracture Prevention with Zoledronate in Older Women with Osteopenia. N Engl J Med. 2018;379(25):2407-2416. [ Links ]
69 Sanderson J, Martyn-St James M, Stevens J, Goka E, Wong R, Campbell F, et al. Clinical effectiveness of bisphosphonates for the prevention of fragility fractures: A systematic review and network meta-analysis. Bone. 2016; 89:52-58. [ Links ]
70 Zhou J, Ma X, Wang T, Zhai S. Comparative efficacy of bisphosphonates in short-term fracture prevention for primary osteoporosis: a systematic review with network meta-analyses. Osteoporos Int. 2016;27(11):3289-3300. [ Links ]
71 Wang G, Sui L, Gai P, Li G, Qi X, Jiang X. The efficacy and safety of vertebral fracture prevention therapies in postmenopausal osteoporosis treatment: Which therapies work best? a network meta-analysis. Bone Joint Res. 2017; 6(7):452-463. [ Links ]
72 Panagiotakou A, Yavropoulou M, Nasiri-Ansari N, Makras P, Basdra EK, Papavassiliou AG, et al. Extra-skeletal effects of bisphosphonates. Metabolism. 2020;110:154264. [ Links ]
73 Lu L, Lu L, Zhang J, Li J. Potential risks of rare serious adverse effects related to long-term use of bisphosphonates: An overview of systematic reviews. J Clin Pharmacy Therap 2020;45:45-51. [ Links ]
74 Rodríguez AJ, Ernst MT, Nybo M, Prieto-Alhambra D, Ebeling PR, Hermann AP, et al. Oral Bisphosphonate use Reduces Cardiovascular Events in a Cohort of Danish Patients Referred for Bone Mineral Density. J Clin Endocrinol Metab. 2020;105(10):3215-3225. [ Links ]
75 Cummings SR, Lui L-Y, Eastell R, Allen IE. Association Between Drug Treatments for Patients With Osteoporosis and Overall Mortality Rates: A Meta-analysis. JAMA Intern Med. 2019;179 (11):1491-1500. [ Links ]
76 Li Y-Y, Gao L-J, Zhang Y-X, Liu S-J, Cheng S, Liu Y-P, et al. Bisphosphonates and risk of cancers: a systematic review and meta-analysis. Br J Cancer. 2020;123 (10):1570-1581. [ Links ]
77 Dömötör ZR, Vörhendi N, Hanák L, Hegyi P, Kiss S, Csiki E, et al. Oral Treatment With Bisphosphonates of Osteoporosis Does Not Increase the Risk of Severe Gastrointestinal Side Effects: A Meta-Analysis of Randomized Controlled Trials. Front Endocrinol (Lausanne). 2020;11:573976. [ Links ]
78 Deng Y, Zhang Z, Jia X, Cheng W, Zhou X, Liu Y, et al. Oral bisphosphonates and incidence of cancers in patients with osteoporosis: a systematic review and meta-analysis. Arch Osteoporos. 2018;14:1. [ Links ]
79 Rizzoli R, Reginster J-Y, Boonen S, Bréart G, Diez-Perez A, Felsenberg D, et al. Adverse reactions and drug-drug interactions in the management of women with postmenopausal osteoporosis. Calcif Tissue Int. 2011;89(2): 91-104. [ Links ]
80 Kim DH, Rogers JR, Fulchino LA, Kim CA, Solomon DH, Kim SC. Bisphosphonates and risk of cardiovascular events: a meta-analysis. PLoS One. 2015;10(4): e0122646. [ Links ]
81 Pijnenburg L, Salem J-E, Lebrun-Vignes B, Sibilia J, Javier R-M, Arnaud L. Atrial fibrillation in patients treated with intravenous zoledronic or pamidronic acid: a pharmacoepidemiological study. Eur J Endocrinol. 2021;154: 437-444. [ Links ]
82 Fuggle NR, Cooper C, Harvey NC, Al-Daghri N, Brandi ML, Bruyere O, et al. Assessment of Cardiovascular Safety of Anti-Osteoporosis Drugs. Drugs 2020;80:1537-1552. [ Links ]
83 Miller PD, Jamal SA, Evenepoel P, Eastell R, Boonen S. Renal safety in patients treated with bisphosphonates for osteoporosis: A review. J Bone Miner Res. 2013;28(10):2049-2059. [ Links ]
84 Evenepoel P, Cunningham J, Ferrari S, Haarhaus M, Javaid MK, Lafage-Proust M-H, et al. European Consensus Statement on the diagnosis and management of osteoporosis in chronic kidney disease stages G4–G5D. Neph-rol Dial Transplant. 2020;36(1):42-59. [ Links ]
85 Khan AA, Morrison A, Hanley DA, Felsenberg D, McCauley LK, O’Ryan F, et al. Diagnosis and management of osteonecrosis of the jaw: a systematic review and international consensus. J Bone Miner Res. 2015;30(1):3-23. [ Links ]
86 López-Delgado L, Riancho-Zarrabeitia L, Riancho JA. Genetic and acquired factors influencing the effectiveness and toxicity of drug therapy in osteoporosis. Expert Metab Toxicol. 2016;12 (4):389-398. [ Links ]
87 Ebeling PR, Akesson K, Bauer DC, Buchbinder R, Eastell R, Fink HA, et al. The Efficacy and Safety of Vertebral Augmentation: A Second ASBMR Task Force Report. J Bone Miner Res. 2019; 34(1):3-21. [ Links ]
88 Black DM, Abrahamsen B, Bouxsein ML, Einhorn T, Napoli N. Atypical Femur Fractures - Review of epidemio-logy, relationship to bisphosphonates, prevention and clinical management. Endocr Rev. 2018;40:333-368. [ Links ]
89 Black DM, Geiger EJ, Eastell R, Vittinghoff E, Li BH, Ryan DS, et al. Atypical Femur Fracture Risk versus Fragility Fracture Prevention with Bisphosphonates. N Engl J Med. 2020; 383(8):743-753. [ Links ]
90 Keren S, Leibovitch I, Ben Cnaan R, Neudorfer M, Fogel O, Greenman Y, et al. Aminobisphosphonate-associated orbital and ocular inflammatory disease. Acta Ophthalmol. 2019;97(5): e792-e799. [ Links ]
91 Jackson C, Freeman ALJ, Szlamka Z, Spiegelhalter DJ. The adverse effects of bisphosphonates in breast cancer: A systematic review and network meta-analysis. PLoS One. 2021;16(2): e0246441. [ Links ]
92 Cummings SR, San Martin J, McClung MR, Siris ES, Eastell R, Reid IR, et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361 (8):756-765. [ Links ]
93 Boonen S, Adachi JD, Man Z, Cummings SR, Lippuner K, Törring O, et al. Treatment with Denosumab Reduces the Incidence of New Vertebral and Hip Fractures in Postmenopausal Women at High Risk. J Clin Endocrinol Metab. 2011;96(6):1727-1736. [ Links ]
94 Bone HG, Wagman RB, Brandi ML, Brown JP, Chapurlat R, Cummings SR, et al. 10 years of denosumab treatment in postmenopausal women with osteoporosis: results from the phase 3 randomised FREEDOM trial and open-label extension. Lancet Diabetes Endocrinol. 2017;5(7):513-523. [ Links ]
95 Cummings SR, Ferrari S, Eastell R, Gilchrist N, Jensen JEB, McClung M, et al. Vertebral Fractures After Discontinuation of Denosumab: A Post Hoc Analysis of the Randomized Placebo-Controlled FREEDOM Trial and Its Extension. J Bone Miner Res. 2018;33: 190-198. [ Links ]
96 Seeto AH, Abrahamsen B, Ebeling PR, Rodríguez AJ. Cardiovascular Safety of Denosumab Across Multiple Indica-tions: A Systematic Review and Meta-Analysis of Randomized Trials. J Bone Miner Res. 2020;36(1):24-40. [ Links ]
97 Meunier PJ, Roux C, Seeman E, Orto-lani S, Badurski JE, Spector TD, et al. The Effects of Strontium Ranelate on the Risk of Vertebral Fracture in Women with Postmenopausal Osteoporosis. N Engl J Med. 2004;350(5): 459-468. [ Links ]
98 Neer RMN, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, Reginster JYI, et al. Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med. 2001;344(19): 1434-1441. [ Links ]
99 Langdahl BL, Silverman S, Fujiwara S, Saag K, Napoli N, Soen S, et al. Real-world effectiveness of teriparatide on fracture reduction in patients with osteoporosis and comorbidities or risk factors for fractures: Integrated analysis of 4 prospective observational studies. Bone. 2018;116:58-66. [ Links ]
100 Díez-Pérez A, Marin F, Eriksen EF, Kendler DL, Krege JH, Delgado-Rodríguez M. Effects of teriparatide on hip and upper limb fractures in patients with osteoporosis: A systematic review and meta-analysis. Bone. 2019;120:1-8. [ Links ]
101 Simpson EL, Martyn-St James M, Hamilton J, Wong R, Gittoes N, Selby P, et al. Clinical effectiveness of denosumab raloxifene, romosozumab, and teriparatide for the prevention of osteoporotic fragility fractures: A systematic review and network meta-analysis. Bone. 2020;130:115081. [ Links ]
102 Geusens P, Marin F, Kendler DL, Russo LA, Zerbini CAF, Minisola S, et al. Effects of Teriparatide Compared with Risedronate on the Risk of Fractures in Subgroups of Postmenopausal Women with Severe Osteoporosis: The VERO Trial. J Bone Miner Res. 2018;33(5): 783-794. [ Links ]
103 Miller PD, Hattersley G, Riis BJ, Williams GC, Lau E, Russo LA, et al. Effect of abaloparatide vs placebo on newvertebral fractures in postmeno-pausalwomen with osteoporosis a randomized clinical trial. JAMA. 2016; 316(7):722-733. [ Links ]
104 Reginster J-Y, Bianic F, Campbell R, Martin M, Williams SA, Fitzpatrick LA. Abaloparatide for risk reduction of nonvertebral and vertebral fractures in postmenopausal women with osteoporosis: a network meta-analysis. Osteoporos Int. 2019;30(7):1465-1473. [ Links ]
105 McClung MR, Grauer A, Boonen S, Bolognese MA, Brown JP, Diez-Perez A, et al. Romosozumab in postmenopausal women with low bone mineral density. N Engl J Med. 2014;370(5):412-420. [ Links ]
106 Cosman F, Crittenden DB, Ferrari S, Khan A, Lane NE, Lippuner K, et al. FRAME Study: The Foundation Effect of Building Bone With 1 Year of Romosozumab Leads to Continued Lower Fracture Risk After Transition to Denosumab. J Bone Miner Res. 2018;33 (7):1219-1226. [ Links ]
107 Saag KG, Petersen J, Brandi ML, Kara-plis AC, Lorentzon M, Thomas T, et al. Romosozumab or Alendronate for Fracture Prevention in Women with Osteoporosis. N Engl J Med. 2017;377 (15):1417-1427. [ Links ]
108 Kaveh S, Hosseinifard H, Ghadimi N, Vojdanian M, Aryankhesal A. Efficacy and safety of Romosozumab in treatment for low bone mineral density: a systematic review and meta-analysis. Clin Rheumatol. 2020;39(11):3261-3276. [ Links ]
109 Michael Lewiecki E, Blicharski T, Goemaere S, Lippuner K, Meisner PD, Miller PD, et al. A Phase III Randomized Placebo-Controlled Trial to Evaluate Efficacy and Safety of Romosozumab in Men With Osteoporosis. J Clin Endocrinol Metab. 2018;103(9):3183-3193. [ Links ]
110 Lv F, Cai X, Yang W, Gao L, Chen L, Wu J, et al. Denosumab or romosozumab therapy and risk of cardiovascular events in patients with primary osteoporosis: Systematic review and meta analysis. Bone. 2020;130:115121. [ Links ]
111 Clark W, Bird P, Gonski P, Diamond TH, Smerdely P, McNeil HP, et al. Safety and efficacy of vertebroplasty for acute painful osteoporotic fractures (VAPOUR): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet. 2016;388(10052):1408-1416. [ Links ]
112 Firanescu CE, De Vries J, Lodder P, Venmans A, Schoemaker MC, Smeet AJ, et al. Vertebroplasty versus sham procedure for painful acute osteoporotic vertebral compression fractures (VERTOS IV): Randomised sham controlled clinical trial. BMJ. 2018;361. [ Links ]
113 Buchbinder R, Osborne RH, Ebeling PR, Wark JD, Mitchell P, Wriedt C, et al. A randomized trial of vertebroplasty for painful osteoporotic vertebral fractures. N Engl J Med. 2009;361:557-568. [ Links ]
114 Kallmes DF, Comstock BA, Heagerty PJ, Turner JA, Wilson DJ, Diamond TH, et al. A randomized trial of vertebroplasty for osteoporotic spinal fractures. N Engl J Med. 2009;361(6):569-579. [ Links ]
115 Lou S, Shi & X, Zhang & X, Lyu & H, Li & Z, Wang Y, et al. Percutaneous vertebroplasty versus non-operative treatment for osteoporotic vertebral compression fractures: a meta-analysis of randomized controlled trials. Osteoporos Int. 2019;30(12):2369-2380. [ Links ]
116 Zhu Y, Cheng J, Yin J, Zhang Z, Liu C, Hao D. Therapeutic effect of kypho-plasty and balloon vertebroplasty on osteoporotic vertebral compression fracture: A systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore). 2019; 98(45):e17810. [ Links ]
117 Diez-Perez A, Adachi JD, Agnusdei D, Bilezikian JP, Compston JE, Cummings SR, et al. Treatment failure in osteoporosis. Osteoporos Int. 2012;23(12): 2769-2774. [ Links ]
118 Kamimura M, Nakamura Y, Ikegami S, Uchiyama S, Kato H, Taguchi A. Significant improvement of bone mineral density and bone turnover markers by denosumab therapy in bisphospho-nate-unresponsive patients. Osteoporos Int. 2017;28(2):559-566. [ Links ]
119 Nogués X, Nolla JM, Casado E, Jódar E, Muñoz-Torres M, Quesada-Gómez JM, et al. Spanish consensus on treat to target for osteoporosis. Osteoporos Int. 2018;29(2):489-499. [ Links ]
120 Thomas T, Casado E, Geusens P, Lems WF, Timoshanko J, Taylor D, et al. Is a treat-to-target strategy in osteoporosis applicable in clinical practice? Consensus among a panel of European experts. Osteoporos Int. 2020;31(12): 2303-2311. [ Links ]
121 Ferrari S, Libanati C, Lin CJF, Brown JP, Cosman F, Czerwiński E, et al. Relationship Between Bone Mineral Density T-Score and Nonvertebral Fracture Risk Over 10 Years of Denosumab Treatment. J Bone Miner Res. 2019;34(6):1033-1040. [ Links ]
122 Compston J, Cooper A, Cooper C, Gittoes N, Gregson C, Harvey N, et al. UK clinical guideline for the prevention and treatment of osteoporosis. Arch Osteoporos. 2017;12(1):43. [ Links ]
123 Eastell R, Rosen CJ, Black DM, Cheung AM, Murad MH, Shoback D. Pharmacological Management of Osteoporosis in Postmenopausal Women: An Endocrine Society* Clinical Practice Guideline. J Clin Endocrinol Metab. 2019; 104(5):1595-1622. [ Links ]
124 Conley RB, Adib G, Adler RA, Åkesson KE, Alexander IM, Amenta KC, et al. Secondary Fracture Prevention: Consensus Clinical Recommendations from a Multistakeholder Coalition. J Bone Miner Res. 2019;35(1):36-52. [ Links ]
125 Camacho PM, Petak SM, Binkley N, Diab DL, Eldeiry LS, Farooki A, et al. American Association of Clinical En-docrinologists/American College of Endocrinology Clinical Practice Guidelines for the Diagnosis and Treatment of Postmenopausal Osteoporosis-2020 Update. Endocr Pract. 2020;26: 1-46. [ Links ]
126 Moro Álvarez MJ, Neyro JL, Castañeda S. Therapeutic holidays in osteoporosis: Long-term strategy of treatment with bisphosphonates. Med Clín. 2016; 146(1):24-29. [ Links ]
127 Sølling AS, Harsløf T, Langdahl B. Treatment with Zoledronate Subsequent to Denosumab in Osteoporosis: a Randomized Trial. J Bone Miner Res. 2020;35(10):1858-1870. [ Links ]
128 Leder BZ, Tsai JN, Uihlein A V, Wallace PM, Lee H, Neer RM, et al. Denosumab and teriparatide transitions in postmenopausal osteoporosis (the DATA-Switch study): extension of a randomised controlled trial. Lancet. 2015;386:1147-1155. [ Links ]
129 Leder BZ, Neer RM, Wyland JJ, Lee HW, Burnett-Bowie S-AM, Finkelstein JS. Effects of teriparatide treatment and discontinuation in postmenopausal women and eugonadal men with osteoporosis. J Clin Endocrinol Metab. 2009;94(8):2915-2921. [ Links ]
130 Guañabens N, Moro-Álvarez MJ, Casado E, Blanch-Rubió J, Gómez-Alonso C, Díaz-Guerra GM, et al. The next step after anti-osteoporotic drug discontinuation: an up-to-date review of se-quential treatment. Endocrine. 2019; 64(3):441-455. [ Links ]
131 Cosman F, Crittenden DB, Adachi JD, Binkley N, Czerwinski E, Ferrari S, et al. Romosozumab Treatment in Postmenopausal Women with Osteoporosis. N Engl J Med. 2016;375(16):1532-1543. [ Links ]
132 Lewiecki EM, Dinavahi RV, Laza-retti-Castro M, Ebeling PR, Adachi JD, Miyauchi A, et al. One Year of Romosozumab Followed by Two Years of Denosumab Maintains Fracture Risk Reductions: Results of the FRAME Extension Study. J Bone Miner Res. 2018; 34(3):419-428. [ Links ]
133 Boonen S, Marin F, Obermayer-Pietsch B, Simoes ME, Barker C, Glass EV, et al. Effects of Previous Antiresorptive Therapy on the Bone Mineral Density Response to Two Years of Teriparatide Treatment in Postmenopausal Women with Osteoporosis. J Clin Endocrinol Metab. 2008;93(3):852-860. [ Links ]
134 Cosman F, Keaveny TM, Kopperdahl D, Wermers RA, Wan X, Krohn KD, et al. Hip and spine strength effects of ad-ding versus switching to teriparatide in postmenopausal women with osteoporosis treated with prior alendronate or raloxifene. J Bone Miner Res. 2013;28(6):1328-1336. [ Links ]
135 Kendler DL, Marin F, Zerbini CAF, Russo LA, Greenspan SL, Zikan V, et al. Effects of teriparatide and risedronate on new fractures in post-menopausal women with severe osteoporosis (VERO): a multicentre, double-blind,double-dummy, randomised controlled trial. Lancet. 2018;391(10117): 230-240. [ Links ]
136 Langdahl BL, Libanati C, Crittenden DB, Bolognese MA, Brown JP, Daizadeh NS, et al. Romosozumab (sclerostin monoclonal antibody) versus teriparatide in postmenopausal women with osteoporosis transitioning from oral bisphosphonate therapy: a randomised, open-label, phase 3 trial. Lancet. 2017;390(10102):1585-1594. [ Links ]
137 Bone HG, Greenspan SL, McKeever c., Bell N, Davidson M, Downs RW, et al. Alendronate and estrogen effects in postmenopausal women with low bone mineral density. J Clin Endocrinol Metab. 2000;85:720-726. [ Links ]
138 Cosman F, Eriksen EF, Recknor C, Miller PD, Guañabens N, Kasperk C, et al. Effects of intravenous zoledronic acid plus subcutaneous teriparatide [rhPTH(1-34)] in postmenopausal osteoporosis. J Bone Miner Res. 2011;26 (3):503-511. [ Links ]
139 Leder BZ, Tsai JN, Uihlein A V, Burnett-Bowie S-AM, Zhu Y, Foley K, et al. Two years of Denosumab and teriparatide administration in postmenopausal women with osteoporosis (The DATA Extension Study): a randomized controlled trial. J Clin Endocrinol Metab. 2014;99(5):1694-1700. [ Links ]
140 Ringe JD, Farahmand P, Faber H, Dorst A. Sustained efficacy of risedronate in men with primary and secondary osteoporosis: results of a 2-year study. Rheumatol Int. 2008;29(3):311-315. [ Links ]
141 Ringe JD, Dorst A, Faber H, Ibach K. Alendronate treatment of established primary osteoporosis in men: 3-year results of a prospective, comparative, two-arm study. Rheumatol Int. 2004; 24(2):110-113. [ Links ]
142 Orwoll ES, Ettinger M, Weiss S, Miller P, Kendler D, Graham J, et al. Alendronate for the treatment of osteoporosis in men. N Engl J Med. 2000;343(9): 604-610. [ Links ]
143 Effect of zoledronic acid therapy on fracture risk in the treatment of men with osteoporosis. N Engl J Med. 2012; 367:1714-1723. [ Links ]
144 Orwoll ES, Miller PD, Adachi JD, Brown J, Adler RA, Kendler D, et al. Efficacy and safety of a once-yearly i.v. Infusion of zoledronic acid 5 mg versus a once-weekly 70-mg oral alendronate in the treatment of male osteoporosis: A randomized, multicenter, double-blind, active-controlled study. J Bone Miner Res. 2010;25(10):2239-2250. [ Links ]
145 Smith MR, Egerdie B, Toriz NH, Feldman R, Tammela TLJ, Saad F, et al. Denosumab in Men Receiving Androgen-Deprivation Therapy for Prostate Cancer. N Engl J Med. 2009;361:745-755. [ Links ]
146 Orwoll E, Teglbjærg CS, Langdahl BL, Chapurlat R, Czerwinski E, Kendler DL, et al. A Randomized, Placebo-Con-trolled Study of the Effects of Denosumab for the Treatment of Men with Low Bone Mineral Density. J Clin Endocrinol Metab. 2012;97(9):3161-3169. [ Links ]
147 Orwoll ES, Scheele WH, Paul S, Adami S, Syversen U, Diez-Perez A, et al. The Effect of Teriparatide [Human Parathyroid Hormone (1-34)] Therapy on Bone Density in Men With Osteoporosis. J Bone Miner Res. 2003;18(1):9-17. [ Links ]
148 Kaufman J-M, Orwoll E, Goemaere S, San Martin J, Hossain A, Dalsky GP, et al. Teriparatide effects on vertebral fractures and bone mineral density in men with osteoporosis: treatment and discontinuation of therapy. Osteoporos Int. 2004;16(5):510-516 [ Links ]
149 Adachi JD, Saag KG, Delmas PD, Liberman UA, Emkey RD, Seeman E, et al. Two-year effects of alendronate on bone mineral density and vertebral fracture in patients receiving glucocorticoids: a randomized, doubleblind, placebo-controlled extension trial. Arthritis Rheum. 2001;44(1): 202-211. [ Links ]
150 Reid DM, Hughes RA, Laan RFJM, Sacco-Gibson NA, Wenderoth DH, Adami S, et al. Efficacy and safety of daily risedronate in the treatment of corticosteroid-induced osteoporosis in men and women: a randomized trial. European Corticosteroid-Induced Osteoporosis Treatment Study. J Bone Miner Res. 2000;15(6):1006-1013. [ Links ]
151 Reid DM, Devogelaer J-P, Saag K, Roux C, Lau C-S, Reginster J-Y, et al. Zoledronic acid and risedronate in the prevention and treatment of glucocorticoidinduced osteoporosis (HORIZON): a multicentre, double-blind, doubledummy, randomised controlled trial. Lancet. 2009;373(9671): 1253-1263. [ Links ]
152 Saag KG, Zanchetta JR, Devogelaer J-PP, Adler RA, Eastell R, See K, et al. Effects of teriparatide versus alendronate for treating glucocorticoid-induced osteoporosis: Thirty-six-month results of a randomized, double-blind, controlled trial. Arthritis Rheum. 2009; 60(11):3346-3355. [ Links ]
153 Glüer CC, Marin F, Ringe JD, Hawkins F, Möricke R, Papaioannu N, et al. Comparative effects of teriparatide and risedronate in glucocorticoid- induced osteoporosis in men: 18-month results of the EuroGIOPs trial. J Bone Miner Res. 2013;28(6):1355-1368. [ Links ]
154 Richy F, Ethgen O, Bruyere O, Reginster J-Y. Efficacy of alphacalcidol and calcitriol in primary and corticoste roid-induced osteoporosis: a metaanalysis of their effects on bone mineral density and fracture rate. Osteoporos Int. 2004;15(4):301-310. [ Links ]
155 Yanbeiy ZA, Hansen KE. Denosumab in the treatment of glucocorticoid-induced osteoporosis: a systematic review and meta-analysis. Drug Des Devel Ther. 2019;13:2843-2852. [ Links ]
156 Wu J, Zhang Q, Yan G, Jin X. Denosumab compared to bisphosphonates to treat postmenopausal osteoporosis: a meta-analysis. J Orthop Surg Res. 2018; 13(1):194. [ Links ]
157 Saag KG, Pannacciulli N, Geusens P, Adachi JD, Messina OD, Morales-Torres J, et al. Denosumab Versus Risedronate in Glucocorticoid-Induced Osteoporosis: Final Results of a TwentyFour-Month Randomized, Double-Blind, Double-Dummy Trial. Arthritis Rheumatol. 2019l;71(7):1174-1184. [ Links ]
158 Florez H, Ramírez J, Monegal A, Guañabens N, Peris P. Spontaneous vertebral fractures after denosumab discontinuation: A case collection and review of the literature. Semin Arthritis Rheum. 2019;49(2):197-203. [ Links ]
Annex I.
Group of SEIOMM experts for the revision of the Osteoporosis Guidelines
Cannata Andía, Jorge. Departamento de Medicina. Universidad de Oviedo. Oviedo.
Cano, Antonio. Servicio de Ginecología y Obstetricia. Hospital Clínico Universitario de Valencia-INCLIVA. Valencia.
Carbonell Abella, Cristina. Centro de Salud Via Roma Barcelona. Universidad de Barcelona. Barcelona.
Casado Burgos, Enrique. Servicio de Reumatología. Hospital Universitari Parc Taulí. Instituto de Investigación e Innovación Parc Taulí. Sabadell (Barcelona).
Ciria Recasens, Manuel. Servicio de Reumatología de Parc de Salut Mar. Barcelona.
Corral-Gudino, Luis. Servicio de Medicina Interna. Hospital Universitario Río Hortega. Valladolid.
del Pino Montes, Javier. Servicio de Reumatología Hospital Universitario Salamanca. Salamanca.
Del Río Barquero, Luis Miguel. CETIR Centro Médico. Barcelona.
Díaz Curiel, Manuel. Enfermedades Metabólicas Óseas. Fundacion Jiménez Díaz. Madrid.
Díez Pérez, Adolfo. Instituto Hospital del Mar de Investigación Médica. Barcelona.
García Vadillo, Alberto. Servicio de Reumatología. Hospital Universitario de la Princesa. Universidad Autónoma de Madrid. Madrid.
Gómez Alonso, Carlos. UGC Metabolismo Óseo. Hsopital Universitario Central de Asturias. ISPA. Universidad de Oviedo. Oviedo.
Gómez de Tejada Romero, María Jesús. Departamento de Medicina. Universidad de Sevilla. Sevilla.
González Macías, Jesús. Departamento de Medicina y Psiquiatría. Universidad de Cantabria. Santander.
Guañabens, Nuria. Servicio de Reumatología. Hospital Clínic. IDIBAPS. Universidad de Barcelona. Barcelona
Hawkins Carranza, Federico. Unidad de Metabolismo Óseo, Instituto de Investigación. Hospital Universitario 12 de Octubre. Madrid.
Jódar Gimeno, Esteban. Departamento de Endocrinología. Quirón Salud Madrid. Madrid.
Malouf Sierra, Jorge. Unidad de Metabolismo Mineral. Departamento de Medicina Interna. Hospital de la Santa Creu i Sant Pau. Barcelona.
Martínez Díaz-Guerra, Guillermo. Servicio de Endocrinología. Hospital Universitario 12 de Octubre. Madrid.
Monegal Brancos, Ana. Servicio de Reumatología. Hospital Clinic Barcelona. Barcelona.
Muñoz Torres, Manuel. UGC Endocrinología y Nutrición. Hospital Universitario Clínico San Cecilio. Universidad de Granada. Granada.
Naves Díaz, Manuel. Unidad de Gestión Clínica de Metabolismo Óseo. Hospital Universitario Central de Asturias. ISPA. RedinREN del ISCIII. Oviedo.
Nogues, Xavier. Departamento de Medicina Interna. Hospital del Mar. Universidad Pompeu Fabra. Barcelona.
Nolla, Joan M. Servicio de Reumatología. IDIBELL-Hospital Universitari de Bellvitge. Barcelona.
Olmos Martínez, José Manuel. Servicio de Medicina Interna. Hospital Universitario Marqués de Valdecilla-IDIVAL. Universidad de Cantabria. Santander.
Pérez-Castrillón, José Luis. Hospital Universitario Río Hortega. Universidad de Valladolid. Valladolid.
Peris Bernal, Pilar. Servicio de Reumatología. Hospital Clinic de Barcelona. Universidad de Barcelona. Barcelona.
Quesada Gómez, José Manuel. Instituto Maimónides de Investigación Biomédica de Córdoba (IMIBIC), Hospital Universitario Reina Sofía. Universidad de Córdoba. Córdoba.
Riancho, José A. Servicio de Medicina Interna. Hospital Universitario Marqués de Valdecilla-IDIVAL. Universidad de Cantabria. Santander.
Rodríguez García, Minerva. Servicio de Nefrología. Hospital Universitario Central de Asturias. Oviedo.
Sosa Henríquez, Manuel. Universidad de Las Palmas de Gran Canaria. Hospital Universitario Insular. Unidad Metabólica Ósea. Las Palmas de Gran Canaria.
Torrijos Eslava, Antonio. Reumatólogo SEIOMM. Madrid.
Valero Díaz de Lamadrid, Carmen. Servicio de Medicina Interna. Hospital Universitario Marqués de Valdecilla. IDIVAL. Santander. Universidad de Cantabria. Santander.











 text in
text in