My SciELO
Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista de Osteoporosis y Metabolismo Mineral
On-line version ISSN 2173-2345Print version ISSN 1889-836X
Rev Osteoporos Metab Miner vol.14 n.1 Madrid Jan./Mar. 2022 Epub Aug 22, 2022
https://dx.doi.org/10.4321/s1889-836x2022000100007
ORIGINALS
Knowledge and clinical decisions of Colombian dentists about the risk of osteonecrosis of the jaws in patients receiving treatment for osteoporosis
1Department of Internal Medicine. Pontificia University Javeriana - University Hospital San Ignacio. Bogotá (Colombia)
2San Ignacio University Hospital. Rheumatology Unit. Bogotá (Colombia)
3Department of Clinical Epidemiology and Bio-statistics. Pontificia Universidad Javeriana. Bogotá (Colombia)
4Javeriana University Pontifical. School of Dentistry. Bogotá (Colombia)
Introduction:
Osteonecrosis of the jaws is a rare, severe adverse reaction associated with the administration of drugs used to treat osteoporosis and cancer, such as bisphosphonates and denosumab. However, many professionals suspend these medications or defer the procedures until they have the referring physician's authorization. This study evaluates the knowledge and attitudes of a group of Colombian dentists regarding the risk of developing maxillary osteonecrosis with the use of bisphosphonates and denosumab.
Methods:
A survey was designed from a focus group that was endorsed by experts. A tool of 30 questions was obtained, which was sent to a group of dentists, maxillofacial surgeons, periodontists and oral rehabilitators affiliated with dental societies through the Survey Monkey software.
Results:
The responses of 187 dentists (42.6% with postgraduate studies) were analyzed. 50.3% of dentists mistakenly considered the use of bisphosphonates an absolute contraindication for major dental procedures and 51.3% believed the same regarding denosumab use. 74.6% of professionals would unnecessarily request approval from the referring physician to schedule procedures in patients receiving bisphosphonates and 43.8% for patients receiving denosumab. Our findings were similar regardless of years of experience or level of education.
Conclusion:
Our results suggest that the respondents had little knowledge as to the risk of developing maxillary osteonecrosis with the use of medications for the management of osteoporosis.
Key words: osteonecrosis of the jaws; bisphosphonates; denosumab; osteoporosis; dentists
Introduction
Maxillary osteonecrosis (ONJ) is a rare severe adverse reaction to drugs used to treat osteoporosis and cancer, such as bisphosphonates and denosumab. This complication consists of the progressive destruction of the mandibular and/or maxillary bone, with exposure of the necrotic bone in the oral cavity, which occurs more frequently with the use of antiresorptive agents in cancer and multiple myeloma1,2.
The risk of ONJ with bisphosphonates and denosumab in osteoporosis therapy is very low, close to 0.01%, as it is a low-dose and short-exposure therapy, unlike when they are used in cancer patients, with a risk of around 1.3%3,4. The prevalence of ONJ in patients receiving long-term oral bisphosphonate therapy was reported to be 0.1% (10 cases per 10,000), which increased to 0.21% (21 cases per 10,000) in patients older than 4 years. bisphosphonate exposure5.
Although the risk of ONJ is very low with the use of bisphosphonates and denosumab in osteoporosis, dental professionals still perceive a high risk of presenting this complication. They frequently request authorization for dental procedures to the prescribing physician, leading to dental complications due to delays in carrying out the procedures or associated with the suspension of treatment for osteoporosis6,7.
This study aims to ascertain the degree of knowledge and the clinical decisions that Colombian dentists make regarding ONJ risk associated with the use of bisphosphonates and denosumab in osteoporosis.
Methods
A survey was designed to assess two areas. The first related to the level of knowledge of dentists regarding the risk of developing ONJ with bisphosphonates and denosumab evaluated with general questions about the topic. The second involved the clinical decisions made by professionals, which was assessed with hypothetical clinical cases. The survey development process initially included a focus group, in which a dental professional, a clinical psychologist and epidemiologist expert in qualitative research, a rheumatologist and two internal medicine residents took part. Some initial questions were proposed that were subsequently submitted to a group of experts for approval and correction. The resulting tool was applied to a group of 30 students in their final year of dentistry studies at the Pontificia Universidad Javeriana (Bogotá) as a pilot test, seeking to evaluate the ease of response and understanding. Their comments were taken into account to make the final adjustments to the survey prior to its application.
The survey was hosted in the SurveyMonkey program (Supplement 1) and sent to dentists, maxillofacial surgeons, periodontists and oral rehabilitation specialists, affiliated with the Colombian Odontological Federation, during the period from October 2019 to August 2020. They were invited to participate by sending registered email by each professional, up to a maximum of 3 times. Professionals who reported no clinical practice for one year and those with exclusive pediatric practice were excluded.
The demographic characteristics of the participants are presented in absolute numbers, proportions or as measures of central tendency and dispersion, depending on the type of variable. The comparison analysis between subgroups was performed using a Chi square test. Statistical analysis was carried out using Stata software (Stata: version 15, TX Stata Corp LLC).
The study was approved by the Ethics and Research Committee of the University Hospital San Ignacio and the Pontificia Universidad Javeriana.
RESULTS
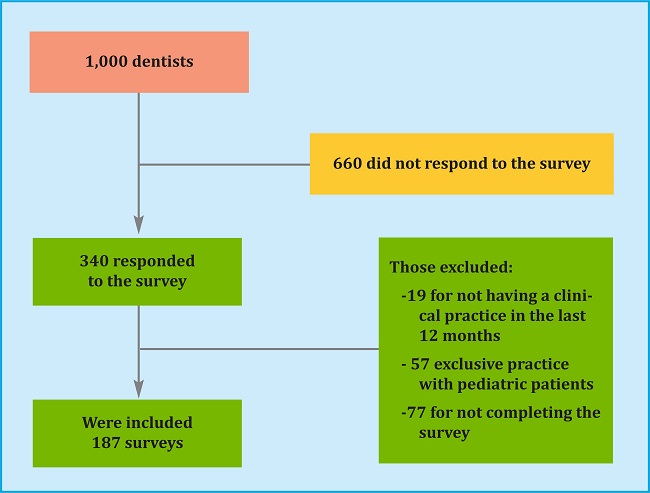
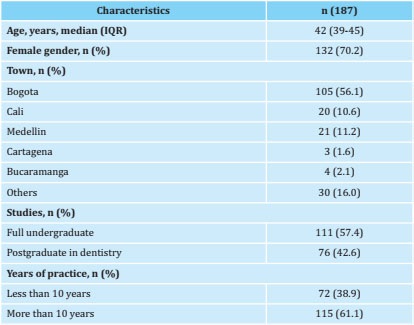
1,000 Colombian dentists were invited to participate. 340 (34%) responded to the survey and of these 19 (5.5%) were excluded because they had no clinical practice in the last 12 months, 57 (16%) due to their exclusive practice with pediatric patients and 77 (22%) because they did not complete the survey. In total, the responses of 187 dentists were analyzed (algorithm 1). The median age was 42 years (interquartile range 39-45). The majority were women (70.2%), with a greater presence of dentists from Bogotá (56.2%). Table 1 shows the demographic characteristics of the group of dentists who took part in the study.
Knowledge assessment
When evaluating the risk of developing ONJ, 50.2% of the respondents considered that the use of BFs was an absolute contraindication for a major dental procedure, while 51.3% expressed the same opinion for denosumab. For minor procedures, 3.2% of those surveyed considered the use of bisphosphonates an absolute contraindication and 27.8% a relative contraindication to carry out the procedure. The percentages for the use of denosumab were 4.2% and 28.3%, respectively (figure 1).
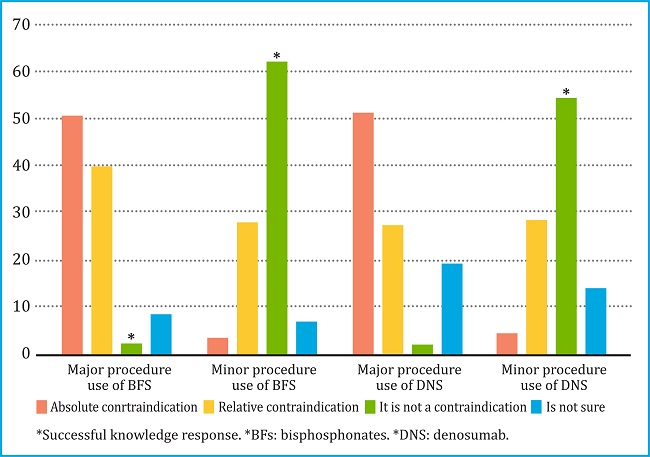
Figure 1. Knowledge of Colombian dentists about the use of bisphosphonates or denosumab due to the risk of osteonecrosis of the jaw, during major and minor dental procedures
41.2% considered that ONJ risk was the same for those receiving bisphosphonates compared to those receiving denosumab. 45% considered that the risk of developing ONJ is greater if bisphosphonates are administered orally. 50% considered that the risk of ONJ is the same in patients with cancer, compared to patients with osteoporosis receiving bisphosphonates or denosumab. 78% of dentists considered that the risk of ONJ increases with the time of exposure to bisphosphonates, and 50% with the time of exposure to denosumab. Regarding the question of the risk of developing ONJ with bisphosphonate compared to denosumab, 70% were not sure.
70% of dentists reported that less than 25% of patients in their clinical practice diagnosed with osteoporosis and 57.8% of these patients being treated with bisphosphonates or denosumab. Of the dentists surveyed, 76.4% have not dealt with any case of ONJ, and of those who had, only 16.9% were associated with osteoporosis. 41.6% are unaware of any document for diagnosing and managing osteonecrosis of the jaw.
Evaluation of clinical decisions
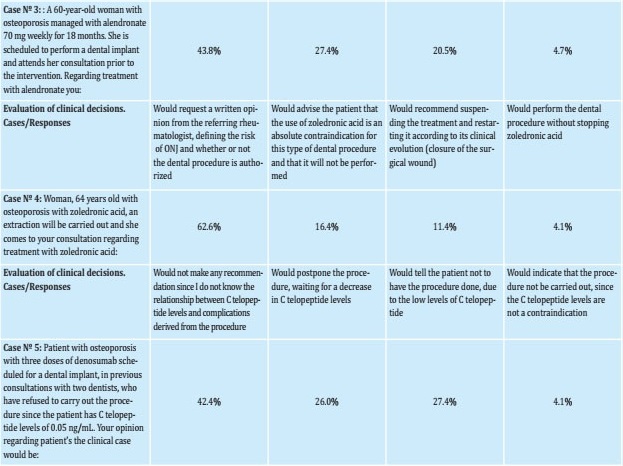
The hypothetical cases used and the clinical decisions that dentists would make in that situation are presented in table 2. Of the reported decisions, the following stood out:
Table 2. Clinical cases concerning decisions taken by Colombian dentists about the use of bisphosphonates or denosumab due to the risk of ONJ, during major and minor dental procedures
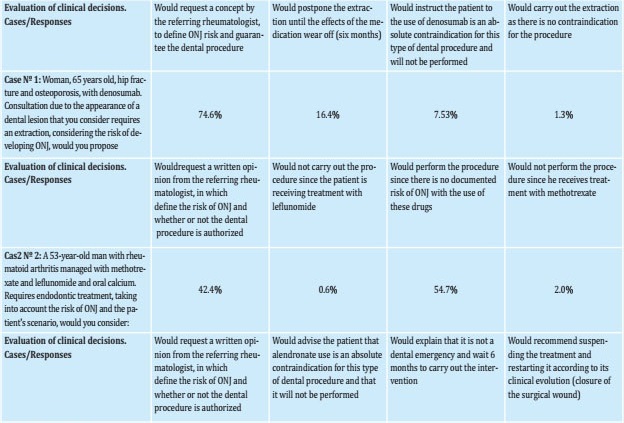
Table 2. (Cont.) Clinical cases concerning decisions taken by Colombian dentists about the use of bisphosphonates or denosumab due to the risk of ONJ, during major and minor dental procedures
For case 1 (65-year-old woman with hip fracture and osteoporosis treated with denosumab for a year who required an extraction), only 1.37% considered carrying out the extraction and 74.66% considered it necessary to request an extraction authorization from the referring physician in order to perform the procedure.
For case 2 (53-year-old man with a history of rheumatoid arthritis managed with methotrexate and leflunomide who requires endodontics), 42.47% would request an opinion from the referring physician.
For case 3 (60-year-old woman with osteoporosis managed with alendronate and pending dental implant), 3.42% considered carrying out the treatment without suspending the bisphosphonate and 43.84% would request authorization from the referring physician to endorse the procedure.
For case 4 (64-year-old woman with osteoporosis undergoing treatment with zoledronic acid who required tooth extraction), 4.1% considered carrying out the procedure without suspending the bisphosphonate and 62.3% would request authorization from the referring physician.
For case 5 (Patient with osteoporosis who is being managed with denosumab, of which he has received 3 doses, with telopeptide C levels at 0.05 ng/mL), 26.03% would postpone the procedure while waiting for the decreased levels of telopeptide C.
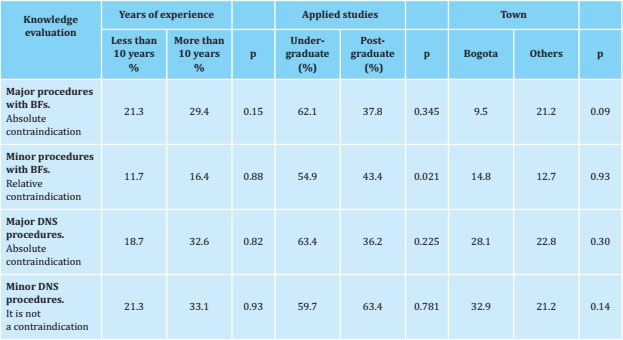
The subgroup analysis showed that a lower proportion of professionals with postgraduate studies considered the use of bisphosphonates a relative contraindication for carrying out minor procedures (43.4 vs 54.9%, p 0.021) (table 3). For the other clinical decisions, no significant differences were found, regardless of the years of experience, the level of education (complete undergraduate vs. postgraduate) or the city where the professional practice was carried out.
Discussion
Colombian dentists' knowledge and attitudes regarding the risk of developing ONJ with the use of bisphosphonates and denosumab, in treating osteoporosis, were analyzed in our study. We found a high proportion of professionals had limited knowledge of the ONJ risk associated with bisphosphonates and denosumab. In this sense, they would make incorrect decisions regarding the scheduling time of major and minor procedures.
Our findings are similar to those reported in other countries, where a low level of knowledge regarding the subject was reported. A study published by R Al-Eid et al. shows the results of a survey of 74 dentists in Saudi Arabia in which 39.2% of the respondents were not familiar with the term ONJ and 54% had no knowledge regarding the diagnosis and treatment of ONJ; 44% were unsure whether to discontinue bisphosphonate therapy prior to tooth extraction8. A 2017 survey of Mexican dentists by Vinitzky-Brener et al. showed that only 40.5% were aware of ONJ and only 24.6% were familiar with some type of bisphosphonate9. Another study assessing the knowledge of dentists about ONJ associated with bisphosphonates carried out in Korea by Yoo et al., in 2010, reported that only 56.5% of those surveyed knew the term ONJ and 31.4% related it to bisphosphonate use10. Similar findings were reported by Alhussain et al., in 2015 in a study conducted with Canadian dentists, where 60% of the respondents did not have sufficient knowledge about ONJ and 50% did not know how to manage it11.
This is the first study conducted to assess the knowledge and clinical decisions of Colombian dentists. Our study suggests that there is a lack of knowledge regarding the risk of ONJ in treating osteoporosis with bisphosphonates and denosumab. According to the American Association of Oral and Maxillofacial Surgeons (AAOMS) 2014 recommendations and the first Colombian Consensus of ONJ associated with medications of 2019, treatment with bisphosphonates or denosumab is not an absolute or relative contraindication. Furthermore, treatment should not be suspended to perform the dental procedure. However, the committee recognizes that there are limited data to support or refute the pharmacological vacation period for patients with osteoporosis treatment, but vacation therapy may be beneficial after prolonged exposure to treatment12,13. This is based on the very low risk of ONJ in the context of osteoporosis, which is 0.01%, as demonstrated by the FREEDOM study, which evaluated the use of denosumab in 4,550 patients, where there were no ONJ cases. In the HORIZON study with 7,765 patients managed with zoledronic acid and followed up for 3 years, only one case of ONJ occurred13,14.
50% of the dentists responded that the risk of ONJ is the same in patients with cancer compared to osteoporosis. Studies that have evaluated ONJ risk in both scenarios have shown a large difference in risk, which is 10 to 150 times higher in cancer compared to osteoporosis (0.1-1.5% vs 0.01%)15,16. Regarding knowledge of scientific documents for the prevention and management of patients with ONJ, 41% are unaware of document for ONJ treatment. There are two important documents for the diagnosing and treating ONJ, the Guide of the American Association of Oral and Maxillofacial Surgeon (AAOMS) of 2014 and the I Colombian Consensus of ONJ published in 201912,17. In the study by R Al-Eid et al., the authors reported that most respondents were unaware of the AAOMS guidelines17,18.
In the evaluation of attitudes carried out through clinical scenarios, in clinical cases Nº 1 and 3 of patients with osteoporosis managed with denosumab and bisphosphonates, respectively, 74.66% and 43.84% would request an opinion from the referring physician to authorize the dental procedure. According to the AOMMS recommendations and the Colombian ONJ consensus, an assessment by the referring physician is not required to define dental treatment12,17.
Furthermore, our study suggests that ONJ is a very low frequency disease in dentistry, as shown by the fact that 76.5% of those surveyed have not had any case of ONJ and 70.2% of dentists respondents have less than 25% of patients in their clinical practice diagnosed with osteoporosis. Of these patients with osteoporosis, 57.9% are treated with bisphosphonates and denosumab. Our study suggests that the respondents lack knowledge for decision-making regarding the risk of ONJ with the use of bisphosphonates and denosumab in treating osteoporosis.
This study has some limitations. The sample size was relatively small, which may not be representative of all dentists in our country. However, the study encompasses the highest number of dentists participating in knowledge assessment, compared with other previous studies that used a similar methodology.
Conclusion
The results of our study suggest that there is limited knowledge regarding the risk of developing ONJ with the use of bisphosphonates and denosumab in the treatment of osteoporosis. This low level of knowledge impacts the dental care of patients with osteoporosis managed with bisphosphonates or denosumab, by suspending therapy or delaying dental procedures. A greater effort is required to educate undergraduate and postgraduate students. Updating educational programs for graduated dentists could identify the actual risk and factors associated with ONJ in patients with osteoporosis treated with bisphosphonates or denosumab.
REFERENCES
1 Kuroshima S, Sasaki M, Sawase T. Medication-related osteonecrosis of the jaw: A literature review. J Oral Biosci 2019;61(2):99–104. [ Links ]
2 Reid IR, Cornish J. Epidemiology and pa-thogenesis of osteonecrosis of the jaw. Nat Rev Rheumatol 2012;8(2):90-96. [ Links ]
3 Marx, Y. Sawatari, M. Fortin, V. Brou-mand, Bisphosphonate-induced expo-sed bone (osteonecrosis/osteopetrosis) of the jaws: risk factors, recognition, prevention, and treatment, J. Oral Maxillofac. Surg. 63 (2005) 1567-1575. [ Links ]
4 García-Martínez L, Martín-Payo R, Pelaz-García A, Sierra-Vega M, Junquera-Gutiérrez LM. Intervención para la mejora del conocimiento de los factores de riesgo para el desarrollo de osteonecrosis maxilar en pacientes a tratamiento con bisfosfonatos. Ensayo clínico aleatorizado. Enferm Clin. 2017, Enfcli.2017.04.001 [ Links ]
5 Ruggiero SL, Dodson TB, Fantasia J, Goodday R, Aghaloo T, Mehrotra B, et al. American association of oral and maxillofacial surgeons position paper on medication-related osteonecrosis of the jaw - 2014 update. J Oral Maxillofac Surg 2014;72(10):1938–1956. [ Links ]
6 Vermeer JAF, Renders GAP, Everts V. Osteonecrosis of the Jaw—a Bone Site-Specific Effect of Bisphosphonates. Curr Osteoporos Rep. 2016;14(5): 219-225. [ Links ]
7 Kim SH, Lee YK, Kim TY, Ha YC, Jang S, Kim HY. Incidence of and risk for osteonecrosis of the jaw in Korean osteoporosis patients treated with bisphosphonates: A nationwide cohort-study. Bone. 2020;(May):115650. [ Links ]
8 Al-Eid R, Alduwayan T, Bin Khuthaylah M, Al Shemali M. Dentists' knowledge about medication-related osteonecrosis of the jaw and its management. Heliyon 2020;6(7):e04321. [ Links ]
9 Vinitzky-Brener, N.G. Ibañez-Mancera, A.M. Aguilar-Rojas, AP Alvarez-Jardon, Knowledge of bisphosphonate-related osteonecrosis of the jaws among Mexican dentists, Med. Oral Patol. Oral Cir. Bucal 22 (2017) e84–e87. [ Links ]
10 J.Y. Yoo, Y.D. Park, Y.D. Kwon, D.Y. KimY, J.Y. Ohe, Survey of Korean dentists on the awareness on bisphosphonate-re-lated osteonecrosis of the jaws, J. Investig. Clin. Dent. 1 (2010) 90–95. [ Links ]
11 A. Alhussain, S. Peel, L. Dempster, C. Clokie, A. Azarpazhooh, Knowledge, practices, and opinions of Ontario dentists when treating patients recei-ving bisphosphonates, J. Oral Maxillofac. Surg. 73 (2015) 1095–1105 [ Links ]
12 Chalem, Monique; Diaz, Nohemi, Or-juela Adriana, Gonzalez D. I consenso colombiano de osteonecrosis de los maxilares asociada a medicamentos (omam). Asoc Colomb Osteoporos y Metab Miner. 2019;1:25. [ Links ]
13 Cummings SR, Martin JS, McClung MR, Siris ES, Eastell R, Reid IR, et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis, N Engl J Med, 2009 Aug 20;361(8):756-765. [ Links ]
14 Dennis M Black 1 , Pierre D Delmas, Richard Eastell, Ian R Reid, Steven Boo-nen, Jane A Cauley, Felicia Cosman, Péter Lakatos, Ping Chung Leung, Zu-lema Man, Carlos Mautalen, Peter Me-senbrink, Huilin Hu, John Caminis, Karen Tong, Theresa Rosario-Jansen, Joel Krasnow, Trisha F Hue, Deborah Sellmeyer, Erik Fink Eriksen, Steven R Cummings, Randomized controlled trial Once-Yearly Zoledronic Acid for Treatment of Postmenopausal Osteoporosis, HORIZONT study New England Journal, 2007 May 3;356(18):1809-1822. [ Links ]
15 Raje N, Terpos E, Willenbacher W, Shi-mizu K, García-Sanz R, Durie B, et al. Denosumab versus zoledronic acid in bone disease treatment of newly diag-nosed multiple myeloma: an international, double-blind, double-dummy, randomised, controlled, phase 3 study. Lancet Oncol. 2018;19(3):370–381. [ Links ]
16 Vahtsevanos K, Kyrgidis A, Verrou E, Ka-todritou E, Triaridis S, Andreadis CG, et al. Longitudinal cohort study of risk factors in cancer patients of bisphospho-nate-related osteonecrosis of the jaw. J Clin Oncol. 2009;27(32):5356-5362. [ Links ]
17 Ruggiero SL. Diagnosis and Staging of Medication-Related Osteonecrosis of the Jaw. Oral Maxillofac Surg Clin North Am 2015;27(4):479–487. [ Links ]
18 Filleul O, Crompot E, Saussez S. Bis-phosphonate-induced osteonecrosis of the jaw: A review of 2,400 patient cases. J Cancer Res Clin Oncol. 2010;136(8):1117–1124. [ Links ]
Annex 1.
Annex 1 Survey
-
Have you had patients in the last 12 months ?
-
Does your clinical practice work exclusively with children under 18?
-
Gender
Age: years
-
City of clinical practice
-
Level of study attained
-
Years of clinical practice:
Knowledge evaluation:
Note to start the survey: In the following survey, the term bisphosphonate refers to the following drugs: zoledronic acid, iban-dronate, alendronate, risedronate. These drugs, such as denosumab, are therapeutic options for osteoporosis treatment.
-
When carrying out major dental procedures (exodontics, open-field surgical procedures) in patients with osteoporosis treated with bisphosphonates, the use of this type of medication is:
-
When carrying out minor dental procedures (root canal treatment, cleaning, prophylaxis, resins, amalgams and crowns) in patients with osteoporosis treated with bisphosphonates, the use of this type of medication is::
-
When carrying out major dental procedures (extractions, open-field surgical procedures) in patients with osteoporosis under treatment with denosumab, the use of this medication is:
-
When carrying out minor dental procedures (root canal treatment, cleanings, prophylaxis, resins, amalgams and crowns) in patients with osteoporosis treated with denosumab, the use of this medication is::
-
The risk of developing osteonecrosis of the jaw in patients with osteoporosis with the use of bisphosphonates compared to the use of denosumab is:
-
The risk of developing osteonecrosis of the jaw in patients with osteoporosis who receive bisphosphonates according to their route of administration is:
-
The risk of developing osteonecrosis of the jaw in patients with osteoporosis receiving bisphosphonates:
-
The risk of developing osteonecrosis of the jaw in patients with osteoporosis receiving denosumab:
-
The risk of developing osteonecrosis of the jaw in patients with osteoporosis receiving bisphosphonates or denosumab compared to patients receiving these same therapies for cancer treatment is:
-
Are you aware of published scientific consensus documents for the prevention and management of patients with drug-induced osteonecrosis of the jaw?
-
The risk of developing drug-induced osteonecrosis of the jaw in a patient with osteoporosis treated with bisphosphonates or denosumab is:
-
Approximately, what percentage of patients in your clinical practice have a diagnosis of osteoporosis?
-
Approximately, what percentage of patients in your clinical practice have been diagnosed with osteoporosis and are being treated with bisphosphonates or denosumab?
-
How many cases of drug-induced osteonecrosis of the jaw have you seen in the last 12 months?
-
Of these seen cases of drug-induced osteonecrosis of the jaw, most were related to:
-
Do you know if there is any diagnostic test to assess the risk of osteonecrosis of the jaw in patients receiving bisphosphonates or denosumab?
Clinical cases-clinical decisions
-
65-year-old woman, history of hip fracture and osteoporosis, treated with denosumab for 1 year (last application 1 month ago). Consultation due to appearance of dental lesion that you consider requires extraction, considering the risk of developing ONJ, you would propose:
Postpone the extraction until the effect of the medication ends (six months)
Carry out the extraction as there is no contraindication for the procedure*
Request a written concept from the treating rheumatologist, in which the risk of ONJ is defined and whether or not the dental procedure is authorized
Advise the patient that the use of denosumab is an absolute contraindication for this type of dental procedure and will not be carried out
-
A 53-year-old man with a history of rheumatoid arthritis managed with methotrexate 10 mg/week and leflunomide 20 mg daily, does not use glucocorticoids. His rheumatologist also prescribed oral calcium at his last visit. He requires endodontic treatment, taking into account the risk of ONJ and the patient's scenario, you:
Would not carry out the procedure as the patient is treated with methotrexate
Would not carry out the procedure as the patient is treated with leflunomide
Would carry out the procedure as there is no documented ONJ risk with the use of these medications*
Would request a written concept from the treating rheumatologist, in which the risk of ONJ is defined and whether or not the dental procedure is authorized
-
A 60-year-old woman with a history of osteoporosis managed with alendronate 70 mg weekly for 18 months. She is scheduled to perform a dental implant and attends her consultation prior to the intervention. Regarding treatment with alendronate, you:
Would perform the dental procedure without discontinuing alendronate*
Would recommend suspending the treatment and restarting it according to its clinical evolution (closure of the surgical wound)
Would explain that it is not a dental emergency and I would wait a 6-month cleaning time to perform the intervention
I would advise the patient that the use of alendronate is an absolute contraindication for this type of procedure dental and will not be performed
Would request a written concept from the treating rheumatologist, in which the risk of ONJ is defined and whether or not the dental procedure is authorized
-
A 64-year-old woman with a history of osteoporosis who has been managed with zoledronic acid 5 mg intravenously every year for two years, is scheduled for the next application in two months. The patient will undergo an extraction and attends your consultation prior to the intervention. Regarding treatment with zoledronic acid you:
Would carry out the dental procedure without stopping zoledronic acid*
Would recommend suspending the treatment and restarting it according to its clinical evolution (closure of the surgical wound)
Would explain to him that it is not a dental emergency and I would wait a year for cleaning to carry out the intervention
Would advise the patient that the use of zoledronic acid is an absolute contraindication for this type of dental procedure and will not be performed
Would request a written concept from the treating rheumatologist, in which the risk of ONJ is defined and whether or not the dental procedure is authorized
-
A patient comes to your consultation who wants a second dental opinion. The patient suffers from osteoporosis and as treatment has received three doses of denosumab in the last two years, she was scheduled to carry out a dental implant, however, in previous consultations with two dentists, they have refused to perform the procedure as the patient presents C telopeptide levels at 0.05 ng/mL. Your opinion regarding the clinical case of the patient:
Would indicate that the procedure be carried out, since the levels of C telopeptide are not a contraindication*
Would tell you not to have the procedure done, due to raised C telopeptide levels
Would postpone the procedure, until C telopeptide levels decrease
Would not make any recommendations as I do not know the relationship between C telopeptide levels and complications derived from the procedure
Received: July 01, 2021; Accepted: January 13, 2022











 text in
text in