Highlight
Histamine is emerging as an important mediator in irritable bowel syndrome, although the pathophysiological role of histamine is not entirely clear.
Using a virtual simulator, it was possible to determine the antagonistic effect of mepyramine on the contractile action of histamine. In addition, the pKB parameter found was of higher potency compared to other antihistamines.
The use of agents targeting histamine receptor antagonists, such as mepyramine, it can be used as an option for the treatment of patients with irritable bowel syndrome, thus offering greater therapeutic options.
Introduction
Histamine is a short-time action endogenous amine1 widely distributed in both neural and non-neural compartments including several tissues like skin, mucosa of the bronchial tree and the gastrointestinal tract, it is predominantly stored into mast cells and basophils2,3. Histamine exerts its biological actions by binding to 4 subtypes of histamine receptors (HRs), which are named chronologically in order of their discovery: H1R- H4R1,4. These receptors belong to the G protein-coupled receptor superfamily and produce their functional response through different mechanisms, H1 receptors function via Gq/G11-mediated activation of phospholipase C and Ca2+ mobilization, while H2 receptors are positively coupled to adenylate cyclase via Gs, leading to the formation of cAMP. Finally, the signal transduction pathway used by the H3 receptor and H4 receptors appears to be dependent on Gi and Go proteins and producing the inhibition of adenylyl cyclase, calcium mobilization from intracellular stores and stimulation of MAP kinase in both heterologous expression systems and native immune cells5.
The effects of histamine in the gastrointestinal tract are complex and can be neurally- mediated or direct effects4. Different receptor subtypes are involved: H1 receptors mediating permeability, contraction and motility of smooth muscle cells1,4, as well as vascular dilatation and modulation of visceral pain1, H2 receptors mediate both muscle relaxation and neurally-induced contraction, H3 receptors inhibit transmitter release from different types of neurons4, and H4 receptors impact on gastrointestinal contractility and motility and modulation of visceral pain1. However, the dominant effect of histamine is contraction, which masks the other effects4.
Irritable bowel syndrome (IBS) is a functional, multifactorial disease characterized by exacerbated gastrointestinal motor reflexes responses6. The typical symptoms are abdominal pain and change in bowel habits. Bloating, tenesmus, bowel urgency, and abdominal distension can also occur1.
Histamine is emerging as an important mediator in IBS. Although the pathophysiological role of histamine in IBS is not entirely clear, it has been associated with symptom severity in IBS1,7. Different studies indicated that mucosal biopsy supernatants from patients with IBS contained higher levels of histamine compared to biopsy supernatants from healthy volunteers7. Moreover, the evidence indicates that overproduction of histamine may be responsible for diarrhea caused by increased neuronal secretomotor function1. In the same way activation of H1 receptors in the intestinal smooth muscle is associated with constriction which results in intestinal cramps and diarrhea8.
The use of HR antagonists is emerging as a potential therapeutic option in patients with IBS. A recent study showed that blocking H1R with ebastine, a second generation H1R antagonist, attenuates the visceral hypersensitivity and may ameliorate other IBS symptoms could be improved. In addition, the H1R antagonist ketotifen has been shown to reduce some IBS symptoms1,7.
The aim of this study was to verify the antagonistic effect of mepyramine, an H1R antagonist4) and tubocurarine, a muscle relaxant that blocks nerve impulses9, in the contractile action of histamine in a guinea pig ileum, to identify a potential therapeutic agent to treat of IBS. For this purpose, the simulation program Virtual Organ Bath 16 V 2.8 was used due to its simplicity and versatility to quantify the change in strength (g) by the contraction of the smooth muscle in a quick way in response to the application of a variety of agonist and antagonists. To define the competitive natura of the antagonist and calculation of the affinity constant, the Schild method was used.
Methods
This study used the program “Organ Bath Simulation 16 V 2.8” as a virtual platform to simulate the contraction in the smooth muscle of the guinea pig ileum. The change of force (g) produced by the relaxation and or contraction was quantified in direct relation to the addition of histamine in the presence and absence of mepyramine and tubocurarine in a Krebs-Henseleit solution (10 ml) (Figure 1).
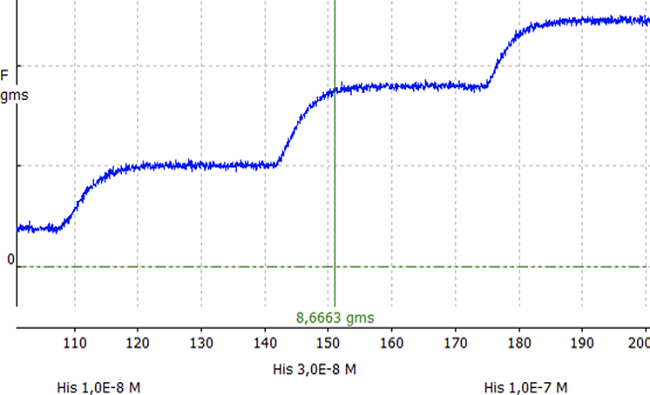
Figure 1. Experimental procedure used to measure contraction force(g) using the program Organ Bath Simulation 16 V 2.8
The contractile effect of histamine was determined in an accumulative manner by increasing the concentration of histamine in 0.5 log units slots (-11 to -4), obtaining the control curve. Intermediate concentrations were used in order to appreciate in a better way the contractile effect of histamine after each addition; the process was performed in triplicate in order to standardize the data.
The antagonistic effect of the selected substances was determined adding increasing concentrations of mepyramine (Final concentration: 10-10M to 10-7M) and tubocurarine (Final concentration: 10-7M to 10-4M) to the solution of the organ bath, in separated test. The solution of the organ bath was pre-incubated with a definite concentration of the antagonist and the contractile effect of histamine was determined in accumulative manner as previously described. The process was performed in triplicate for each concentration of both antagonists.
An exhaustive manual identification of the maximum contraction obtained for each of the added concentrations of histamine was performed. The values obtained were recorded in the Excel 2017 calculation program.
Concentration-response curves of the contractile effects of histamine on smooth muscle of the guinea pig ileum in the absence and presence of two possible antagonists were obtained. The contractile force data were exported to the GraphPad Prism® graphic program in order to obtain the concentration-response curves through the non-linear regression model indicated below.
In addition, pharmacodynamic parameters (EC50, Emax, Hill slope) were extracted from the program, as well as information of statistical relevance for the analysis and understanding of the data.
Statistical analysis:
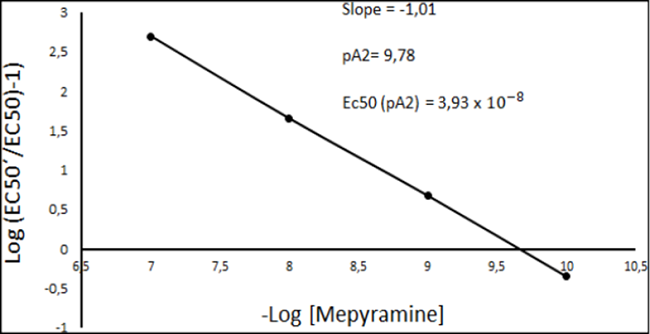
Data are expressed as mean± SD. Student´s t- test for unpaired data was used to determine the statistical significance of the difference between groups through the comparison of the values Emax and EC50. P < 0.05 was considered to be significant. Determination of the antagonist competitive nature and calculation of the affinity constant (pKB) was performed. Mepyramine and tubocurarine competitiveness analysis was performed through the Schild method using Excel 2017. The linear regression of the data was made to determine the competitive nature of the antagonist (Slope) and the affinity constant (pKB) (Extrapolating the line on a Schild plot to zero on the y-axis).
Results
Effect of histamine in the presence of antagonists on the contraction of the smooth muscle of the guinea pig ileum.
The concentration-response curves obtained with each of the antagonist concentrations are shown in Figure 2. As seen in the control curve, histamine (10-11M to 10-4M) causes contraction of the smooth muscle of the guinea pig ileum with an EMax of 14.09 ±0.20 and a pEC50 of 7.007±0.042. The Hill Slope was calculated as 1.096±0.94. The estimated quadratic function for the contractile effect of histamine in the absence of an antagonist was defined as:
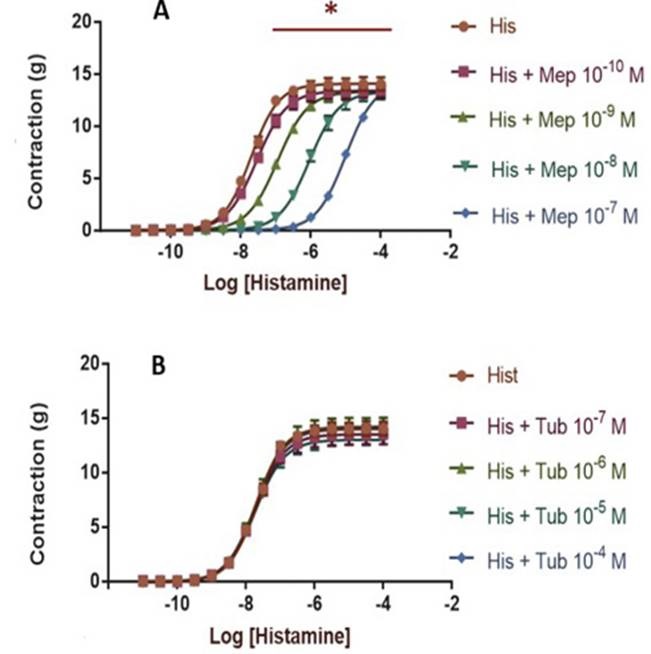
Figure 2. Concentration response curves of contractile effect of histamine in a slice from the ileum region of a guinea pig in presence of Mepyramine (A) and Tubocurarine (B). Each curve represents a single experiment performed in triplicate and points represent means with SEM shown by vertical bars. *P < 0.05 EC50 (Student's unpaired t-test).
The contractile effect of histamine was antagonized by mepyramine to the concentrations 10-10M-10-7M producing a statistically significant increase in pEC50 (P <0.05) at the studied concentrations. A statistically significant decrease in EMax was also evidenced (Table 1) for concentrations ranging from 10-10M to 10-8M.
Table 1. Parameters of the concentration-response curves of histamine in the presence of mepyramine.
| His | His + Mep-10 M | His + Mep-9 M | His + Mep-8 M | His + Mep-7 M | |
|---|---|---|---|---|---|
| pEC50 | 7.707±0.042 | 7.547±0.051 | 6.95±0.038 | 6.044±0.044 | 5.007±0.026 |
| Value P pEC50 | - | 0.0010* | < 0.0001* | < 0.0001* | < 0.0001* |
| EMax | 14.09±0.20 | 13.41±0.22 | 13.35±0.20 | 13.34±0.29 | 14.22±0.31 |
| Value P EMax | - | 0.0015* | 0.0008* | 0.0020* | 0.2698 |
| Hill Slope | 1.096±0.094 | 1.003±0.0966 | 1.007±0.081 | 1.021±0.097 | 1.044±0.0451 |
| R square | 0.9966 | 0.9956 | 0.9975 | 0.9964 | 0.9992 |
*P < 0.05 (Student's unpaired t-test). The R-Square value indicate the experimental points are coupled to the non-linear regression model obtained from GraphPad Prism®
On the other hand, the analysis of the contractile effect of histamine in the presence of tubocurarine (10-7M - 10-4M) indicates that there is no a significant change in muscle contraction in the presence of this substance. In the case of the PEC50 value, statistics were not found a significant variation (P> 0.05) in any of the concentrations used. In the case of EMAX, a statistically significant decrease for concentrations 10-7M and 10-5M was identified with respect to the curve control (Table 2).
Table 2. Parameters of the concentration-response curves of histamine in the presence of tubocurarine.
| His | His + Tub-7 M | His + Tub-6 M | His + Tub-5 M | His + Tub-4 M | |
|---|---|---|---|---|---|
| pEC50 | 7.707±0.042 | 7.712±0.065 | 7.704±0.061 | 7.728±0.044 | 7.726±0.028 |
| Value P pEC50 | - | 0.8310 | 0.8940 | 0.2909 | 0.2544 |
| EMax | 14.09±0.20 | 13.52±0.28 | 14.21±0.28 | 13.02±0.18 | 14.01±0.13 |
| Value P EMax | - | 0.0047* | 0.2962 | 0.0002* | 0.3021 |
| Hill Slope | 1.013±0.0547 | 1.003±0.0966 | 1.007±0.081 | 1.021±0.097 | 1.044±0.0451 |
| R square | 0.9966 | 0.9926 | 0.9934 | 0.9966 | 0.9987 |
*P < 0.05 (Student's unpaired t-test). The R-Square value indicate the experimental points are coupled to the non-linear regression model obtained from GraphPad Prism®
Determination of the competitive nature of the antagonist and calculation of the affinity constant (pKB).
Mepyramine was found to (10-10M- 10-7M) exert a competitive antagonism in histamine receptors in ileum region of a guinea pig (Slope= -1.01) (Figure 3). Moreover, it was found that the negative log of the concentration of mepyramine, which produced a 2-fold shift in the concentration- response curve for histamine (pA2 value) was 9.78 (1.66x10-10M). The EC50 for histamine in this case was calculated as 3.93x10-8M. Finally, given that mepyramine is a competitive antagonist, the negative log of the molar concentration which at equilibrium would occupy 50% of the receptors in the absence of agonist (pKB) was assumed to be equal to pA2 value (pKB 9.78).
Discussion
In the present work it was established through an organ bath simulation that histamine has direct effects on the contractility of the smooth muscle of the intestinal cells isolated from a guinea pig. The Hill slope 1.096±0.94 indicates that there is not cooperativity binding of histamine with HRs.
The contractile effect was antagonized by the H1 antagonist mepiramine, which shows a significant decrease of EC50 (P<0.05) along concentrations range (10-10M - 10-7M). The EC50 decrease implies a decrease of the histamine potency in the contractile effect on smooth muscle in presence of mepyramine. These results are in accordance with the results obtained by Bertaccini G. et al4, who found through in vitro assays that the contractile responses to histamine in longitudinal muscle cells of guinea pig ileum was competitively antagonized by mepyramine 10-8M. Moreover, we found that the presence of the antagonist mepyramine produced a significant (P< 0.05) depression of the maximum response (Emax) to histamine from 10-10M to 10-8M which is also in accordance with the results obtained by Bertaccini G. et al4. In our study, it was found that higher concentrations do not produce depression of the maximum response to histamine, which could be explained for a possible antagonistic effect of mepiramine in the H2R receptors (which mediate relaxation) at high concentrations. However, this information has not been supported by any other study.
From Schild method it was found that mepyramine (10-10M - 10-7M) presents a competitive antagonism by the histamine receptors in the ileum region of a guinea pig (Slope= -1.01). This result is supported by previous investigations performed in isolated isolated intestinal smooth muscle cells4 and guinea- pig left atrial membranes10 where in vitro assays revealed that mepyramine is a competitive inhibitor of histamine in H1R. Since mepyramine was found to be a competitive antagonist of HR, pKB was assumed to be equal to pA2 value =9.78. This result is supported by previous investigation perform by Arunlakshana et. al.11 who calculated the pA2 value of the antagonist effect of histamine by mepyramine on the contractility of a guinea-pig ileum through the method of Schild, with a value of 9.46. Given that pA2 value is a measure of the potency of an antagonist, the pA2 value obtained in our study was compared with the pA2 value reported for other HR antagonists (diphenhydramine: 8.02 and pethidine: 6.13)11. Mepyramine presents higher potency than other antihistamines make it a possible potential antagonistic substance.
On the other hand, the contractile effect of histamine on smooth muscle contractility was not antagonized by tubocurarine. There was not a significant decrease of EC50 (P>0.05) along the concentration range (10-4M - 10-7M). In the same way, it was not possible to perform Schild analysis for the antagonist effect of tubocurarine, due to the similarity EC50 values of histamine in presence and absence of tubocurarine. These results are explained considering that tubocurarine acts as a competitive inhibitor of the nicotinic acetylcholine receptor9. Moreover, according to the results obtained in this investigation it is possible to conclude that there is not any interaction between tubocurarine and HRs and the effect of histamine is not modified in any way in presence of tubocurarine.
From literature review, if the study had been done simulating the real conditions of the organism (in vivo studies), potentiation of the contractile effect of histamine could have been found considering that tubocurarine may release histamine by direct mast cell degranulation, which may result in potentiation of systemic effects of histamine12. This result allows to identify limitations associated with virtual simulations (as used in this research). However, through this method it is possible to identify potential therapeutic options to be able to continue in a next phase of experimentation through in vivo assays.
Due to that there is not congruence in the statistically significant differences found in the Emax at two of the concentrations studied (10-6M and 10-4M). These results were associated with experimental (human) errors presented during the data collection. In a future occasion human factors should be taken into consideration.
Conclusions
The present results provide evidence on the competitive antagonism of mepyramine (10-10M to 10-7M) on the contractile effect of histamine, in smooth muscle from a guinea pig. Moreover, through calculation of pKB parameter, it was found that this compound presents higher potency that other antihistamines, and could be a potential therapeutic agent in IBS. Further research is needed to confirm the contribution and clinical significance of this results. Moreover, it will be necessary to continue performing studies in vivo to establish efficacy and safety of this competitive antagonist.