My SciELO
Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Journal of Negative and No Positive Results
On-line version ISSN 2529-850X
JONNPR vol.5 n.12 Madrid Dec. 2020 Epub Dec 18, 2023
https://dx.doi.org/10.19230/jonnpr.3990
ORIGINAL
Functional foods as an alternative to increase the consumption of dietary fiber and proanthocyanidins. Possible effects on the gut microbiota
1Departamento de Nutrición y Ciencia de los Alimentos. Facultad de Farmacia. Universidad Complutense de Madrid. España
2Instituto de Investigación Sanitaria del Hospital Clínico San Carlos (IdISSC). España
Introduction.
Dietary fiber is an essential nutritional component, which, its modulating action on gut microbiota must be outlined. The consumption of foods of vegetable origin, and therefore, the dietary´s fiber consumption, in Spain, as well as in some other countries, has been reduced. Functional foods have been found to be a great food alternative to solve this dietary deficiency.
Objectives.
1. To review dietary´s fiber concept. 2. To get to know the real consumption situation of dietary fiber in Spain, in other European countries; in different age groups. 3. To study the main effects related to dietary fiber consumption, particularly the effects of proanthocyanidins (PA) with respect to the gut microbiome. 4. To revise functional foods concept, and the possibilities of incorporating dietary fiber and PA into different highly consumed foods.
Materials and methods:
The main research support has been the PubMed database, although it has also been used Google Scholar, ResearchGate and SciELO. At the same time scientific articles, books and reports from reliable and corroborated sources have been revised. In addition, official documents have been consulted, as the 2018 Spanish Foods Consumption Report, published by the Spanish Ministry of Agricultura, Pesca y Alimentación; the AESAN website, and the current food regulations.
Results.
It is proved that the real dietary fiber intakes in different European countries, including in Spain, are far below the dietary reference intakes (DRI), in most of the studied age ranges. In this article, there are proposed strategies to achieve these DRI, particularly adding dietary fiber into highly consumed foods (i.e. meat matrices). The concept of functional foods is reviewed, and some of the most relevant publications reporting the effects of PA in relationship to the gut microbiome recovery, and its change to another related to the healthy people´s microbiome. The mechanisms by which the gut microbiome is able to hydrolyse the PA, and consequently release metabolites with gut and systemic protective activity, is also discussed.
Conclusions.
Given that modifying the population consumption habits seems a difficult task, the alternative of formulating functional foods enriched with dietary fiber and PA it is suggested. The increasing positive evidence observed derived from the consumption of functional meat products to which carob fruit fiber has been added, suggests the relevance of continuing investigating in this field, and therefore start the formulation of new functional foods (i.e. cereals, creams, sweets, etc.) enriched with this PA-enriched fiber, which would lead to an adequate dietary fiber consumption and the benefits from its functional functions.
Keywords Dietary fiber; dietary´s fiber consumption; dietary fiber recommended dietary intakes; gut microbiome; proanthocyanidins; carob-fruit
Abreviations
CVD, cardiovascular diseases
T2DM, type 2 diabetes mellitus
DRI, dietary reference intakes
FAO: Food and Agriculture Organization
PA, proanthocyanidins
WHO, World Health Organization
Introduction
Among food components, dietary fiber has raised special awareness during the last decades(1-3). It is worth wide to remember that in the classic texts, fiber was not included as a nutrient, as it was considered that could not be absorbed, and subsequently could not provide energy to the body. Nonetheless, nowadays it is known that dietary fiber has beneficial effects for the consumer, especially on reducing the risk of degenerative diseases as cardiovascular diseasea (CVD), obesity, diabetes mellitus or cancer(1-6). One of the most novel aspects is the dietary fiber activity on the gut modulatinge several digestive system functions, as maintaining the appropriate integrity and functionality of the mucous membrane, principally the colonic one. In addition, its fermentability contributes to the global dietary energy, and allows maintaining the whole gut microbiome diversity and functionality, that in turn is responsible for creating an ideal environment to make easier the nutrient´s entry and the selective waste and pathogenic microorganisms elimination(3,6,7) .
Dietary fiber
In 2011, the Association of Cereal Chemists defined dietary fiber as “the edible parts of plants or carbohydrates analogous that are resistant to digestion and absorption in the human small intestine with complete or partial fermentation in the large intestine. Dietary fiber includes polysaccharides, oligosaccharides, lignin, and associated plant substances(8). Later the Codex Alimentarius(9) pointed out that the decision of including or not three to nine monomeric units of carbohydrates in to the definition of dietary fiber, is the responsibility of each country competent authorities.
Dietary fiber is composed of several components (Table 1) that explain why it cannot be considered a chemical identity with well-defined functions. Dietary fiber classification is usually made attending to its most relevant properties:
Table 1. Dietary fiber classification and its relationship with the kind of carbohydrates and associated compounds.
| CARBOHYDRATES | ASSOCIATED SUBSTANCES | ||||
|---|---|---|---|---|---|
| SOLUBLE FIBER | INSOLUBLE FIBER | ||||
| FERMENTABLE FIBER | LITTLE FERMENTABLE FIBER | ||||
| Oligosaccharides (prebiotics) | Starchy polysaccharides | Non-starchy polysaccharides |
Insoluble nonstarchy polysaccharides |
Cutin Lignin Phytates Protein Tannins Ca2+, Na+, Mg2+ |
|
| FOS GOS | Resistant starch | β-glucans Gums Hemicellulose Mucilages Pectins |
Cellulose Hemicellulose | ||
FOS, fructooligosaccharides; GOS, galactooligosaccharides. Modified from Ruiz-Roso(10).
- “Solubility into hydroalcoholic solutions"
- “Hydration degree"
- “Gel formation capacity"
- “Capacity of increasing solutions viscosity"
- “Capacity of being fermented by the colonic bacteria"
- “Chemical structure"
One of the most relevant properties of most of the dietary fiber types, classified as “soluble", is their capacity of producing “highly viscous solutions", also known as “gel formation capacity". These properties determine the gastric emptying and the transit time in the gut. The gastric emptying delay leads to a “satiety feeling", and significantly affects postprandial glycemia and lipemia, as well as the cholesterolemia(1). Furthermore, this kind of dietary fibers can easily be “fermented by the gut microbiome", which produces a rise of the microbial colonic abundance, contributing to increase the faecal mass(1,6,7,9).
On the other hand, those fibers classified as “insoluble" present less capacity for producing viscous solutions, even though some of them present a great ability of “retain water into the gut final section" which directly influences the “faecal weight and volume". In addition, these type of fibers are usually little fermented leading to a faecal mass with bigger waste amounts(1,10).
Dietary fiber and gut microbiome
To understand the existing relationship between dietary fiber consumption and the gut microbiome, it is necessary to understand what fiber fermentation is referring to. Since dietary fiber is not hydrolysed by the digestive enzymes, afterwards it is fermented in the colon essentially by the colonic bacteria activity. Subsequently, they release hexoses, pentoses, and alcohols, products which absorption is not possible in the gut, and therefore continue being broken down by these bacteria populations, in order to release other final products as hydrogen, methane, carbon dioxide, lactic acid, short-chain fatty acids, including acetic, propionic and butyric, among others; and energy that is used by the same bacteria(6,7,11). As a consequence, dietary fiber “fermentability" determines two relevant aspects for the human being: on one hand its prebiotic effect as it boosts colonic bacteria populations growth, and on the other the active metabolites liberation with beneficial effects for the host(1,3,6,7,11).
During the last decade, the interest for gut microbiota has highly increased. It is constituted by yeasts, viruses, fungus, protozoa, bacteriophages, and mainly by bacteria. Thus, the most well-known effects on the gut microbiota are referred to its constituents bacteria(3,6,7).
Recent publications clearly stated that the gut microbiome of healthy people is associated with a bigger microbial diversity in comparison with the gut microbiome of people with pathologies(12-14). The term “microbial diversity" refers to two different aspects: on the one hand the number of species, and on the other the general abundance of each microbial specie(13). Simpson and Campbell(14) have noticed that an adequate dietary fiber intake significantly affects the gut microbiome composition. In addition a high dietary fiber intake is directly associated with a bigger gut microbiota stability, abundance and diversity(6,7,15); whereas a low dietary fiber intake is associated with noticeable imbalances on the gut microbiome, also known as dysbiosis. These imbalances can lead to serious health problems(6,7,12), as they can induce inflammatory processes that are associated with the development of some infectious illnesses, asthma, allergy, obesity, metabolic syndrome, inflammatory bowel disease, type 2 diabetes mellitus (T2DM), liver diseases and colon cancer(1,3).
Additionally, the microbial distribution seems to be clearly related to nutritional aspects. Thus, it has been observed that the gut microbiome of people consuming adequate amounts of dietary fiber looks alike to the gut microbiome of healthy adults; and on the contrary, people whose dietary fiber intake is below the recommended amount, their gut microbiome resembles that of people affected by carcinomas, adenomas, inflammatory bowel diseases, obesity, etc.(12).
At present, it is considered as beneficial bacteria for the human being those whose function improves the intestinal development, just like the digestibility, the immunity or the resistance to enteric pathogenic infection(3). Many studies have highlighted the role of bacteria modulating relevant physiological activities, as normal host digestive functions, immune system maturation acquiring defensive capacity against pathogenic microorganisms, intestinal homeostasis(3,14-16) and vital biological functions in the human body, as brain functionality, mRNA translation, ATP production or glucose metabolization(12).
Nowadays, the routes by which the gut microbiome induce these effects are not completely known; thus, some possible routes have been suggested: a) the colonisation increase of favourable bacteria on the large bowel, able to compete against pathogenic microorganisms; b) the energy production from the fiber fermentation useful for the intestinal cell wall; c) the short-chain fatty acids production; d) the increase of faecal volume; e) modulation of the intestinal transit; f) modulation of the immune system; g) gene expression; and h) cell differentiation in the intestinal wall(3).
The interindividual variability together with the variety of diets used in several studies, make it impossible to formulate dry-off conclusions about the intestinal microbiota properties. However, there is growing evidence of the differences between the predominant bacterial genus in the intestine of healthy and sick individuals, as well as the different endogenous and exogenous factors, that promote or inhibit its development, variety and dysbiosis degree.
It is commonly accepted that the intestinal bacterioma of any healthy individual contains hundreds of species, and although there are major interindividual differences(6,14), four dominant phylum have been identified: Bacteroidetes, Firmicutes, Actinobacteria y Proteobacteria(6,7,12).
More than the 90% of healthy people’s intestinal bacteria belong to Bacteroidetes y Firmicutes phylum, this is the reason why the bacteria of these phyla are normally related to “health sign". The intestinal microbiome of healthy individuals is associated with a bigger proportion of Bacteroidetes, rather than Firmicutes, whereas this correlation is the opposite in the microbiome of obese individuals. Several reviews agree that the most abundant bacteria genus in the intestinal microbiome are Bacteroides, Prevotella, Bifidobacterium, Lactobacillus, Ruminococcus, Caprococcus, Dorea, among others(12,14,15).
The consumption of fermentable fiber enhance the presence of Bifidobacterium which is considered beneficial due to the antiinflammatory effect they have(14). This genus belongs to the Actinobacteria phylum. Other authors confirmed the correlation existing between a higher consumption of fiber from legumes and a higher abundance of this phylum at intestinal level(16). Besides, a higher intake of dietary fiber has been associated to a higher abundance of some types of Clostridia (Firmicutes phylum), such as Lachnospira, Faecalibacterium and SMB53(16). These authors emphasize the beneficial physiological role that some bacteria from the Clostridia genus play, due to the resulting metabolites produced, on the fiber fermentation and its beneficial role on colonocytes. It is also worth mentioning the relationship between a higher consumption of fiber and a higher abundance of Faecalibacterium prausnitzii, a specie with important effects on the production of some interleukins (e.g. IL-10), which has been associated with anti-inflammatory activities(16). It has also been suggested a greater risk of suffering intestinal diseases as Chron disease or colorectal cancer in patients showing a reduction of these species(16).
On the other hand, high fiber intakes have been associated with a reduction of the presence of other bacterial genus such as Actinomyces and Odoribacter(16). Human intestine samples collected from individual colorectal cancer colonoscopies showed a higher prevalence of the Odoribacter genus compared to the healthy ones(16). Similarly, an increased presence of bacteria from Actinomyces genus was found in faecal samples from colorectal cancer subjects(16). Furthermore, numerous studies suggest that diets characterized by a low fiber content result in a higher dysbiosis prevalence with an increment of the Proteobacteria phylum bacteria(17).This includes adherent invasive bacteria, mucosa-associated bacteria and pathogenic bacteria, such as Escherichia Coli(14).
Fiber dietary Reference intakes
Due to the importance of dietary fiber, the sanitary authorities have stablished its dietary reference intakes (DRI), in order to make the population adjust to them. That way, as globally, in Europe and Spain it has been stablished that people should consume at least 25 grams of dietary fiber a day (Table 2).
Table 2. Global and European reference dietary intakes
| AUTHORITY | DRI | EFFECTS |
|---|---|---|
| EFSA, scientific panel NDA | 25 g/day | Normal laxation |
| EFSA, scientific panel NDA | > 25 g/day | Reduce CVD, T2DM risk Improve weight maintenance |
| WHO, FAO/WHO | > 25 g/day |
CVD, cardiovascular diseases; EFSA, European Food Safety Authority; FAO: Food and Agriculture Organization; NDA, EFSA Scientific panel of Dietary Products, Nutrition and Allergies; T2DM, type 2 diabetes mellitus; WHO, World Health Organisation.
The European Commission requested to the Panel of Dietary Products, Nutrition and Allergies an opinion about the dietary fiber DRI, for the European population. The panel established that 25 grams a day are adequate for adults in order to achieve a normal laxation, and at the same time affirms that higher quantities are able to reduce the risk of suffering coronary diseases, T2DM, and improve the maintenance of an adequate corporal weight(18,19). In the same way, the WHO in 2003, recommends the intake of same quantities, an opinion that became supported again in 2007 by the Scientific Update FAO/WHO(18). In Spain the final Nutritional Objectives were set at >14 grams of dietary fiber per 1.000 kcal consumed, which corresponds to > 25 grams/day of dietary fiber in women and >35 grams/day of fiber in men(20) (Table 3).
Tabla 3. Spanish Nutritional objectives
| AUTHORITY | NUTRITIONAL OBJECTIVES | EFFECTS |
|---|---|---|
| SENC | >14 g/1.000 kcal = >25 g/day women >35 g/day men |
Normal growth Adequate laxation |
SENC, Sociedad Española de Nutrición Comunitaria. Modified from Arija et al.(20)
The scientific evidence needed to establish the dietary fiber DRI for children is limited; therefore, it is considered that its consumption should be based on the adults fiber DRI after adjusting it to the energy intakes. It is established that kids should take 2 grams of dietary fiber/MJ of total dietary energy, because this promotes the normal growth and development, besides being an adequate quantity for the right laxation(18) (Table 4).
Table 4. European reference dietary intakes for children aged 1 to 17 years
| AUTHORITY | DRI | EFFECTS | AGE | g/day |
|---|---|---|---|---|
| EFSA | 2 g/MJ energy = 8.4 g/1.000 kcal |
Normal growth Adequate laxation |
From 1 a 3 years | 10 |
| From 4 a 6 years | 14 | |||
| From 7 a 10 years | 16 | |||
| From 11 a 14 years | 19 |
Modified from European Food Safety Authority(18). MJ, Megajoule.
Current dietary fiber consumption
Knowing the relevance of consuming dietary fiber, and the amount needed to take advantage of its benefits, we have investigated what is actually being its population´s consumption. In 2010, EFSA(18) carried out individual surveys in order to know dietary fiber consumption differences among European countries (Table 5).
Table 5. Average European and Spanish dietary fiber consumption in different age groups.
| AGE (YEARS) | DIETARY FIBER INTAKE | DRI | ADJUSTED INTAKES? |
|---|---|---|---|
| Europe | |||
| 1-3 | >10 g/day | >10 g/day | YES |
| 4-10 | 14-16 g/day | 14-16 g/day | YES |
| 11-19 | </>19-21 g/day | 19-21 g/day | IN SOME COUNTRIES |
| 19-34 | <25 g/day | 25 g/ day | NO |
| 34-65 | <25 g/day | 25 g/ day | NO |
| Spain | |||
| 10-14 | Male children 18.5 g/day Female children 17.5 g/day |
19 g/day | NO |
| 15-18 | Male children 18.9 g/day Female children 16.2 g/day |
21 g/day | NO |
| 19-64 | Men 19.2 g/day Women 16.9 g/day |
25 g/day | NO |
Source, European Food Safety Agency, EFSA(18).
In Europe, only children between 1 and 10 years, adjust their dietary fiber intakes to the DRI, in most of the studied countries. Between 11 and 19 years, half of the studied countries have a “consumption below the recommendations", and among those older than 19 years, only Germany, Norway and Poland equalise or exceed the DRI, whereas the other European countries have a far lower dietary fiber consumption(18).
When talking about the consumption of dietary fiber in Spain, it is observed that it is lower than the intakes set at the Nutritional objectives. Not one of the studied ages groups reaches the recommended nutritional objectives and, furthermore, it has been observed that the consumption values become lower as the age gets bigger(20).
Strategies to reach the Reference Dietary Intakes
Due to the low dietary fiber intake in most populations, it seen needed to increase is consumption. Among the proposed strategies, the most important one is to modify present wrong dietary habits; nonetheless, to change it is considered a difficult task. Therefore, other different strategies need to be formulated. Including dietary fiber as an ingredient into every-day consumed foods, is becoming increasingly popular. This has positively influenced the formulation of functional foods. Currently, scientists are working in the incorporation of dietary fiber coming from carob-fruit extract into meat products, as it is rich in proanthocyanidins (PA). The consumption of this kind of meat products produced positive effects on the lipoproteins profile and the very-low-density-lipoprotein-receptors levels negatively induced by atherogenic diets in rats. Its consumption also resulted into positive effects on the insulin signalling of diabetic rats; on the fat digestion, and therefore in the postprandial lipemia of healthy rats. It also decreased the carbohydrates absorption and induced antioxidant activity(21-25), among other effects (Table 6). Due to the current debate about the consumption of meat products(26), scientists are looking for some other foods into which the addition of dietary fiber would be possible.
Tabla 6. Gastrointestinal and systemic metabolic beneficial effects derived from proanthocyanidins consumption.
| Effect on carbohydrates metabolism | Effect on lipids metabolism | Antioxidant effects | Physiological effects | Beneficial effects on chronic pathologies |
|---|---|---|---|---|
| Inhibition of pancreatic αamylase and αglucosidase activity(23,24) | LDL cholesterol level reduction(31) | Pro-oxidant/ antioxidant balance modulation(21) | Bigger faecal excretion with a higher fat content(21) | CVD(30) |
| Partial inhibition of glucose transporters in the small bowel: SGLT1 and GLUT2(23,24) | Lipoproteins profile improvement(30) | Inflammatory processes modulation (21,30) | Right peristalsis stimulation (colloidal gel formation)(31) | Depressive behaviour(30) |
| Reduction of the postprandial hyperglycaemic response and favours a better functioning of the βcells(21) | Lipid-lowering properties: lipid, lipoproteins and fatty tissue metabolism adjustment(21) | Prevent from the formation of carcinogenic nitrosamines(31) | Efficacy against ulcers, child’s diarrhea, intestinal infection and gastrointestinal disorders and pathologies prevention(22,30,31) | CVD, hypertension and microand macro-vascular complications prevention(21,30) |
| Favours a better insulin secretion and reduces the insulin resistance(21) | Free radicals inactivation(31) | Bigger satiety feeling and body weight control (30,31,32) | Obesity, metabolic syndrome(30) | |
| Modulation of metabolic sequences of carbohydrate metabolism, delaying pathologies such as T2DM(30) |
Inhibition of induced lipid peroxidation and suppression of DNA fragmentation(30) | Organism unfavourable compounds capture in the bowel(31) | T2DM(30) | |
| Chronic diseases prevention(30,32) | Dyslipidaemia(30) | |||
| Increased mucin concentrations that favours the intestinal barrier integrity(30) | UV erythema and wound cicatrisation (30) | |||
| Increase secretory IgA: preventing from pathogens entry(30) | Inflammatory bowel disease and ulcerous colitis(30) | |||
| Lung inflammation(30) | ||||
| Cancerous processes(30) |
Between the parentheses the bibliographic references number where the effect is indicated. CVD: Cardiovascular diseases; GLUT2, glucose transporter-2, IgA, immunoglobulin A; LDL, low density lipoproteins; SGLT1, sodium-glucose linked transporter-1, T2DM: type 2 diabetes mellitus; UV: ultraviolet.
Functional foods are those which possess a component, nutrient or not, with a selective effect over one or some body functions, and with an added effect above its nutritional value. These positive effects justify that their functional or even healthy character can be demonstrated(27). These foods must:
Respond to the characteristics of food, strictly speaking. At no-time should they be presented as a pharmaceutical drug. Moreover, they must be natural origin ingredients. • Be consumed within a regular diet.
Exert a specific function in the organism, that enables the improvement of any specific corporal process, or that avoids illnesses risk or worsening(27).
According to Celada(27) and Celada and Sánchez-Muniz(28) a functional food must be obtained from a traditional one by:
Removing a food component with an unfavourable physiological effect.
Increasing the concentration of a food component with favourable physiological effect.
Adding a component with favourable physiological effects that the traditional food does not initially had.
Increasing the bioavailability of one or some of its beneficial components.
Partially replacing an ingredient with unfavourable physiological effects by other(s) with favourable physiological effect
Mixture of some the mentioned previously items.
In order to be considered functional foods, they should be tested to verify that they exert healthy effect once consumed(28). The verification not only should be based on statistic results but also on its importance from a biological point of view(28).
Proanthocyanidins
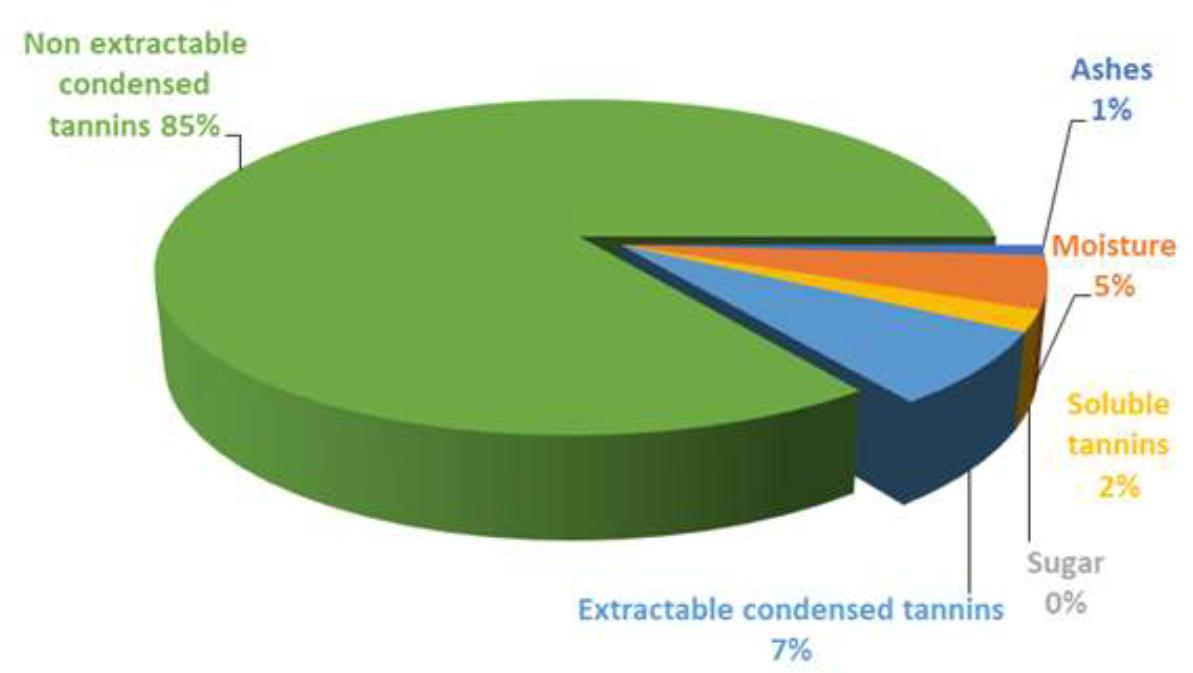
As it has been already discussed, several associated compounds are included into the dietary fiber definition (Table 1). The proanthocyanidins (PA) are polyphenolic compounds which belong to the flavonoids group, and to the flavonols subcategory. The main difference between these polyphenolic compounds and others, is that because of its poor absorption, they reach the colon intact, being able to exert its effects there(30). Macho-González et al.(30) and Alzate Tamayo et al.(31) indicate that due to the multitude of beneficial effects that presents the PA consumption, there is increasingly interest in their study and that of PA-enriched functional foods. The fiber with a higher percentage of polyphenolic compounds is that of carob-fruit(31). This fruit is obtained from the European carob tree, Ceratonia siliqua L. belonging to the Fabaceae family. This is the most known variety in Spain. The high nutritional value of the carob fruit pulp lies in its insoluble or non-extractable antioxidant polyphenols(25). Figure 1 shows the average composition of a purified carob-fruit extract in which abounds the soluble tannins and the soluble and insoluble condensed tannins.
This insoluble polyphenol fraction is also known as condensed tannins. It is composed of polymeric PA of high molecular weight(24). At the same time, the PA are made up of flavan-3ol units. These units are formed by a) a dimethylpropane skeleton (C6-C3-C6) and b) two aromatic rings (A y B) joined by an oxidized heterocycle integrated by three carbon atoms(30,32) (Figure 2).
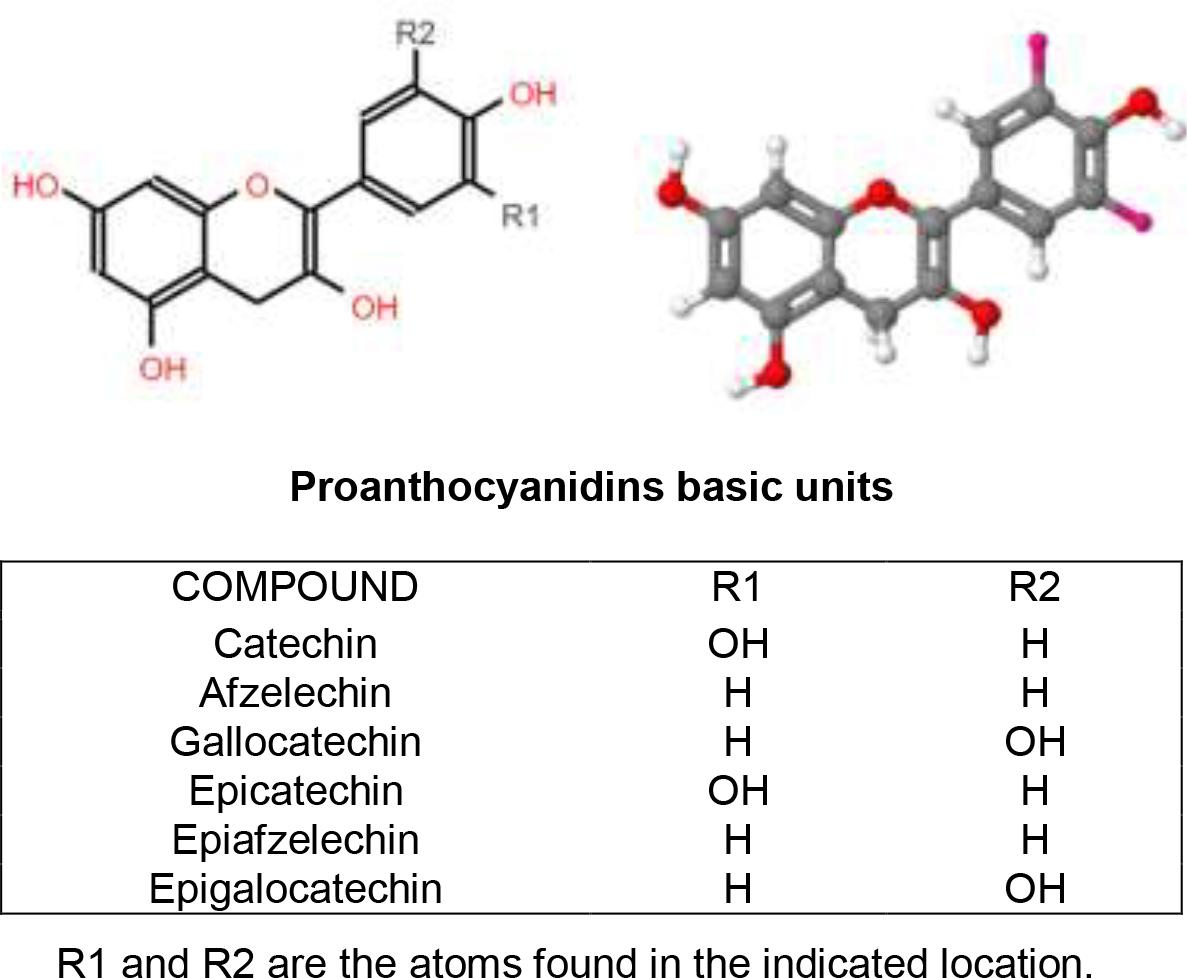
Figure 2. The proanthocyanidin basic chemical structure in two and three dimensions, respectively. Modified from Macho-González et al.(30).
In the Table 6 there are presented some relevant studies about the PA effects.
In addition to the beneficial effects derived from the consumption of PA indicated in table 6, the effects of PA on the intestinal microbiota are being actively studied. The commonly accepted theory about the metabolism of PA is that they reach the colon intact, where the intestinal microbiota depolymerizes and ferments them, producing simpler phenolic acids, whose absorption and phase II metabolism are possible(30). After PA consumption, 10% of their phenolic constituents are found in the colon after 72 hours. This suggests that the biotransformation they undergo is high, although not complete, and that, in these three days, those compounds are in direct contact with the colonic barrier, exerting antioxidant, antiinflammatory, and anti-tumoral properties.
In fact, PA keep almost intact in the small intestine but are hydrolyzed, reduced, decarboxylated, dehydroxylated and demethylated by the colonic bacteria in the large intestine, which gives rise to metabolites of lower molecular weight(30). Among the bacteria present in the human intestinal microbiota, the role of some species as Lactobacillus, Clostridium, Eubacterium, and Bacteroides fermenting PA stands out. These species generate during PA fermentation a series of active metabolites, which act in different body tissues. The main ones appear to be 3-hydroxyphenylacetic, 3-hydroxyphenylpropionic, 3,4-dihydroxyphenylacetic, benzoic acids and 3-hydroxyphenyl-γ-valerolactone(30,32).
Regarding the effect of PAs on the colonic microbiota of animals in physiological situations, it has been found that Firmicutes is the bacterial phylum of the intestinal microbiome that suffers greater modifications(30). As mentioned above, the presence of this bacteria phylum in the intestinal microbiome is accepted to be a health sign, provided that they are found in a lower proportion than those of the Bacteroidetes one. According to Choy et al.(33) the Lactobacillus genus increases, as well as the proportion of the Lachnospiraceae and Ruminococcacceae families from the Clostridales class. Also Han et al.(34) suggest in their studies that the consumption of extracts rich in PA induced similar modifications of the colonic bacteria observing an increase in the family Ruminococcacceae and in the genus Clostridium, although a reduction of the genus Lactobacillus was also observed. The decrease of this genus was also described by Hereu et al.(35) and Lacombe et al.(36) who mentioned the lower abundance of Lactobacillus plantarun after the consumption of grape seed extracts. Lacombe et al.(36) reported the significant reduction in the abundance of bacteria of the Enterococcus genus belonging to the phylum Firmicutes. Queipo-Ortuño et al.(37), on the contrary, found an increase in the latter genus and in Eubacterium, both belonging to the same phylum.
The abundance of Bacteroidetes is also modified after the consumption of extracts rich in PA which produces an increase, specifically, of the Bacteroidia class(34). Other authors such as Lacombe et al.(36) and Queipo Ortuño et al.(37) demonstrated a significant increase in the relative abundance of various genera of this phylum, and detailed some interesting aspects of the Prevotella genus. An increase in the abundance of this phylum is associated with the reduction of bacteria belonging to the Firmicutes phylum.
Regarding the Actinobacteria, Lacombe et al.(36) demonstrated a significant increase in the genus Bifidobacterium who is normally found in high amounts in faecal samples from healthy individuals consuming high amounts of fiber. This significant increase was also noted by Queipo-Ortuño et al.(37) and Espley et al.(38). However, the opposite effect was detected by Pozuelo et al.(39) and Hereu et al.(35). The presence of some genera of this phylum was associated with beneficial effects, while harmful effects were related to others.
On the other hand, with respect to the effects of PA on the colonic microbiota of animals affected by some degenerative diseases, a significant increase (30%) of the Akkermansia genus, belonging to the Verrucomicrobia phylum was reported in two articles(40,41). This genus is considered beneficial as it contributes to block the increase of inflammatory lipopolysaccharides induced by diets containing high fat and sugar contents, as well as being to reverse the metabolic disorders induced by this type of diet, mimicking the antidiabetic effects of metformin. Therefore, it was suggested that colonic increase of these bacteria could prevent the negative metabolic phenotype associated with the dysbiosis caused by obesity. Furthermore, it has been proposed that it stimulates the induction of antimicrobial defenses.
Different studies(41,42) have investigated the effect of PA on the Firmicutes phylum abundance. Some genera of this filum increased after the consumption of PA but others not. On the one hand, Liu et al.(41), Masumoto et al.(43), Van Hul et al.(44) observed an increase of the Roseburia genus in the intestinal microbiota of mice consuming a PA extract in the frame of a diet rich in fat and sugar, compared to those did not consume said extract. This genus, is found in small quantities in the intestinal microbiota of T2DM patients, and plays an important role for intestinal health due to its antiinflammatory activity. Another genus that increases after the consumption of PA is the Allobaculum, which in addition to being a butyrate-producing specie(44) has a key role in the intestinal integrity. On the contrary, Masumoto et al.(41) have observed a decrease in some families and genus of this phylum, such as Lachnospiraceae and Clostridium. A reduction in the latter genus was also reported by Van Hul et al.(44) who suggest that this is a positive effect, since in those mice consuming a diet rich in fat and sugar, this bacterial genus was increased. Liu et al.(43), in contrast, observed an increase in the abundance of bacterial genus Clostridium, as well as Lee et al.(42) detected an increase in the number of Lactobacillus. Several studies(40-42,44) highlight as a positive effect the decrease of the Firmicutes/Bacteroidetes ratio, as the relative abundance of Firmicutes increases and that of Bacteroidetes decreases in genetically obese mice.
On the other hand, some bacterial genus of the Proteobacteria phylum, considered opportunistic pathogens, such as those belonging to the Desulfovibrionaceae family, appear reduced after the intake of cinnamon and grape extracts rich in PA(44). Lee et al.(42) point out a significant increase in the abundance of this phylum, mainly due to the increase of the Gammaproteobacteria, a class that has been shown to have antimicrobial and antiadherent properties against pathogenic bacteria.
Regarding bacteria of the phylum Actinobacteria, contradictory results have been obtained, since Masumoto et al.(41) speak about the reduction of the relative abundance of bacteria from the Bifidobacterium genus, although, by the contrary, Lee et al.(42), have found an increase in this bacterial genus.
Furthermore, according to the role of PA in the microbiota, intestinal permeability and inflammation(30), a bidirectional relationship between PA and the intestinal microbiota is confirmed. This means that the role of the microbiota is essential to transform PA into bioavailable phenolic compounds but at the same time, PA would promote the development of beneficial bacteria, as well as the growth inhibition of other pathogen microorganisms due to their aforementioned prebiotic, bacteriostatic and even bactericidal effects of PA.
Conclusions and future remarks
There is increasing scientific evidence of the beneficial effects derived from the adequate consumption of dietary fiber.
Dietary fiber rich in PA induce several positive health effects.
In order to promote correct dietary fiber consumption and thus, to take advantage of these beneficial effects, different nutritional societies have established its DRI.
The current intake among populations is much lower than these established DRI.
The addition of fiber rich to diet is a favorable alternative to get recommended intakes.
The incorporation and consumption of carob PA to meat-products has given very interesting results, as PA positively affecting different organ functions and, particularly, colonic microbiota abundance and composition in animals models and humans(21-25).
New approaches and studies are needed to ascertain the convenience of consuming this kind of products in order to improve colonic bacterial composition and, therefore, their complex relationships with many body functions.
As the food-matrix can deeply affect results(27,28), we believe that it is necessary to design new functional foods based on other frequently consumed foods (e.g. cereals, creams, pasta, sweets, etc.)(25, 45-49). Thus, a field of great perspectives is opens, being important to study if the beneficial properties observed for PA included in meat-products are maintained or even increased in other PA-enriched foods, particularly with regard to the modulation of the quantity and variety of the gut microbiota and its derived intestinal and systemic functions. This strategy would allow us on the one hand, ensuring fiber consumption and the incorporation of PA, and on the other a greater offer and plurality of functional foods.
Acknowledgements
This work has been partially granted by the Santander - UCM 2018 research project. Reference code: PR75/18-21603 and by Spanish Project PID2019-103872RB-I00. Adrián Macho-González received a predoctoral fellowship award from the Spanish Ministry of Education, Culture and Sports (FPU15/02759).
REFERENCES
1 Gálvez Peralta J, Rodríguez Cabezas ME, Camuesco Pérez D. Capítulo 4 Fibra dietética. En: Tratado de Nutrición Gil A. ed. 3a. edición. Editorial Médica Panamericana. Barcelona, 2017. [ Links ]
2 Soliman GA. Dietary fiber, atherosclerosis, and cardiovascular disease. Nutrients. 2019; 11(5):1155. [ Links ]
3 Fuller S, Beck E, Salman H, Tapsell L. New horizons for the study of dietary fiber and health: a review. Plant Foods Hum Nutr 2016; 71(1):1-12. [ Links ]
4 Jiménez-Escrig A, Sánchez-Muniz FJ. Dietary fibre from edible seaweeds: Chemical structure, physicochemical properties and effects on cholesterol metabolism. Nutr Res 2000; 20(4):585-598. [ Links ]
5 Sánchez-Muniz FJ. Fibra dietética y salud cardiovascular. Nutr Hosp 2012; 27:40-54. [ Links ]
6 Etxeberria U, Milagro FI, González-Navarro CJ, Martinez JA. Role of gut microbiota in obesity. An Real Acad Farm 2016; 82(special issue): 234-259. [ Links ]
7 Redondo N, Nombela C, Marcos A. La microbiota intestinal y su relación con la obesidad. En: IV y V cursos avanzados sobre obesidad y síndrome metabólico. Sánchez Muniz FJ, Marcos Sánchez A, Martínez Hernández JA. (Organizadores). Real Academia Nacional de Farmacia, Madrid. 2018. pp. 389-411. [ Links ]
8 American Association of Cereal Chemists. The definition of dietary fiber. Cereals Foods World 2001. W-2001-0222-01O. Seattle, WA. [ Links ]
9 Comisión del Codex Alimentarius. Roma (Italia). Informe de la 30a reunión del Comité del Codex sobre nutrición y alimentos para regímenes especiales. Ciudad del Cabo (Sudáfrica). 2009. [ Links ]
10 Ruiz-Roso B. Fibra dietética e inmunidad. En: I Curso Avanzado sobre Inmunonutrición. Marcos A y Sánchez-Muniz FJ (Organizadores), Real Academia Nacional de Farmacia, Madrid, 2017. [ Links ]
11 Vilcanqui-Pérez F, Vílchez-Perales C. Fibra dietaria: nuevas definiciones, propiedades funcionales y beneficios para la salud. Revisión. ALAN 2017; 67(2): 146-156. [ Links ]
12 Davis HC. Can the gastrointestinal microbiota be modulated by dietary fibre to treat obesity? Ir J Med Sci 2018; 187(2):393-402. [ Links ]
13 MetaHIT Consortium, Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, y col. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010; 464(7285):59-65. [ Links ]
14 Simpson HL, Campbell BJ. Review article: dietary fibre-microbiota interactions. Aliment Pharmacol Ther 2015; 42(2):158-179. [ Links ]
15 Tap J, Furet J-P, Bensaada M, Philippe C, Roth H, Rabot S, y col. Gut microbiota richness promotes its stability upon increased dietary fibre intake in healthy adults: gut microbiota richness and dietary fibre intake. Environ Microbiol 2015; 17(12):4954-4964. [ Links ]
16 Lin D, Peters BA, Friedlander C, Freiman HJ, Goedert JJ, Sinha R, Miller G, Bernstein M.A, Hayes R. B, Ahn J. Association of dietary fibre intake and gut microbiota in adults. Br J Nutr 2018; 20(9):1014-1022. [ Links ]
17 Shin N-R, Whon TW, Bae J-W. Proteobacteria: microbial signature of dysbiosis in gut microbiota. Trends Biotechnol 2015; 33(9):496-503. [ Links ]
18 European Food Safety Authority. Scientific opinion on dietary reference values for carbohydrates and dietary fibre. EFSA Journal 2010; 77. [ Links ]
19 García Gabarra A, Castellà Soley M, Calleja Fernández A. Ingestas de energía y nutrientes recomendadas en la Unión Europea: 2008-2016. Nutr Hosp 2017; 34(2):490. [ Links ]
20 Arija V, Pérez C, Martínez de Vitoria E, Ortega RM, Serra-Majem L. Valores de referencia de ingesta dietética y de antropometría en estudios poblacionales. Rev Esp Nutr Com 2015; 21(Supl.1)(2):157-167. [ Links ]
21 Macho-González A, Garcimartín A, López-Oliva M, Ruiz-Roso B, Martín de la Torre I, Bastida S, Benedí J. y Sánchez-Muniz F.J. Can carob-fruit-extract-enriched meat improve the lipoprotein profile, VLDL-oxidation, and LDL receptor levels induced by an atherogenic diet in STZ-NAD-diabetic rats? Nutrients 2019; 11(2):332. [ Links ]
22 Macho-González A, Garcimartín A, López-Oliva ME, Celada P, Bastida S, Benedí J, y Sánchez-Muniz FJ. Carob-fruit-extract-enriched meat modulates lipoprotein metabolism and insulin signaling in diabetic rats induced by high-saturated-fat diet. J Funct Foods 2020; 64:103600. [ Links ]
23 Macho-González A, Garcimartín A, Naes F, López-Oliva ME, Amores-Arrojo A, González-Muñoz MJ, Bastida S, Benedí J. y Sánchez-Muniz FJ. Effects of fiber purified extract of carob fruit on fat digestion and postprandial lipemia in healthy rats. J Agric Food Chem 2018; 6(26):6734-6741. [ Links ]
24 Macho-González A, Garcimartín A, López-Oliva ME, Bertocco G, Naes F, Bastida S, Benedí J. y Sánchez-Muniz FJ. Fiber purified extracts of carob fruit decrease carbohydrate absorption. Food Funct 2017; 8(6):2258-2265. [ Links ]
25 Bastida S, Sánchez-Muniz FJ, Olivero R, Pérez-Olleros L, Ruiz-Roso B, JiménezColmenero F. Antioxidant activity of carob fruit extracts in cooked pork meat systems during chilled and frozen storage. Food Chem 2009; 116(3):748-754. [ Links ]
26 Macho-González A, Garcimartín A, López-Oliva ME, Bastida S, Benedí J, Ros G, Nieto G. Sánchez-Muniz FJ. Can meat and meat-products induce oxidative stress? Antioxidants 2020; 9(7):638. [ Links ]
27 Celada Rodríguez MP. Efectos del consumo de productos cárnicos modificados en sujetos con sobrepeso y dislipemia. Tesis Doctoral. Facultad de Farmacia. Universidad Complutense de Madrid. 2017. [ Links ]
28 Celada Rodríguez P, Sánchez-Muniz FJ. Alimentos funcionales. Rendimiento físico y deporte. En: Nutrición deportiva. Desde la Fisiología a la práctica. Capítulo 30. González Gross M. Ed. Panamericana, Madrid. 2020. [ Links ]
29 Ashwell M. Concepts of functional foods. Brussels; Great Britain: ILSI Europe; 2002. [ Links ]
30 Macho-González A, Garcimartín A, López-Oliva ME, Benedí J, Bastida S, Sánchez-Muniz FJ. Inmunonutrición. Estilo de vida: Papel de las proantocianidinas sobre la microbiota, permeabilidad intestinal e inflamación. 2.a ed. Panamericana, Barcelona; 2020. pp. 245-266. [ Links ]
31 Alzate Tamayo LM, Arteaga González DM, Jaramillo Garcés Y. Propiedades farmacológicas del algarrobo (Hymenaea courbaril Linneaus) de interés para la industria de alimentos. Revista Lasallista Investigación 2009; 5(2):100-111. [ Links ]
32 Saura-Calixto F, Pérez-Jiménez J, Touriño S, Serrano J, Fuguet E, Torres JL, Goñi I. Proanthocyanidin metabolites associated with dietary fibre from in vitro colonic fermentation and proanthocyanidin metabolites in human plasma. Mol Nutr Food Res 2010; 54(7):939-946. [ Links ]
33 Choy YY, Quifer-Rada P, Holstege DM, Frese SA, Calvert CC, Mills DA, LamuelaRaventos R.M, Waterhouse A. L. Phenolic metabolites and substantial microbiome changes in pig feces by ingesting grape seed proanthocyanidins. Food Funct 2014; 5(9):2298-2308. [ Links ]
34 Han M, Song P, Huang C, Rezaei A, Farrar S, Brown MA, Ma X. Dietary grape seed proanthocyanidins (GSPs) improve weaned intestinal microbiota and mucosal barrier using a piglet model. Oncotarget 2016; 7(49):80313-80326. [ Links ]
35 Hereu M, Ramos-Romero S, Busquets C, Atienza L, Amézqueta S, Miralles-Pérez B, Nogués MR, Méndez L, Medina I, Torres JL. Effects of combined d-fagomine and omega-3 PUFAs on gut microbiota subpopulations and diabetes risk factors in rats fed a high-fat diet. Sci Rept. 2019; 9:16628. [ Links ]
36 Lacombe A, Li RW, Klimis-Zacas D, Kristo AS, Tadepalli S, Krauss E, Young R, CH Wu V. Lowbush wild blueberries have the potential to modify gut microbiota and xenobiotic metabolism in the rat colon. PLoS One 2013;8(6). [ Links ]
37 Queipo-Ortuño MI, Boto-Ordóñez M, Murri M, Gomez-Zumaquero JM, ClementePostigo M, Estruch R, Cardona F, Andrés-Lacueva C, Tinahones F.J. Influence of red wine polyphenols and ethanol on the gut microbiota ecology and biochemical biomarkers. Am J Clin Nutr 2012; 95(6):1323-1334. [ Links ]
38 Espley RV, Butts CA, Laing WA, Martell S, Smith H, McGhie TK, y col. Dietary flavonoids from modified apple reduce inflammation markers and modulate gut microbiota in mice. J Nutr 2014; 144(2):146-154. [ Links ]
39 Pozuelo MJ, Agis-Torres A, Hervert-Hernández D, López-Oliva ME, Muñoz-Martínez E, Rotger R, Goñi I. Grape antioxidant dietary fiber stimulates Lactobacillus growth in rat cecum. J Food Sci 2012; 77(2):H59-H62. [ Links ]
40 Anhê FF, Roy D, Pilon G, Dudonné S, Matamoros S, Varin TV, y col. A polyphenol-rich cranberry extract protects from diet-induced obesity, insulin resistance and intestinal inflammation in association with increased Akkermansia spp. population in the gut microbiota of mice. Gut 2015; 64(6):872-883. [ Links ]
41 Masumoto S, Terao A, Yamamoto Y, Mukai T, Miura T, Shoji T. Non-absorbable apple procyanidins prevent obesity associated with gut microbial and metabolomic changes. Sci Rep. 2016; 6:31208. [ Links ]
42 Lee S, Keirsey KI, Kirkland R, Grunewald ZI, Fischer JG, de La Serre CB. Blueberry supplementation influences the gut microbiota, inflammation, and insulin resistance in high-fat-diet-fed rats. J Nutr 2018; 148(2):209-219. [ Links ]
43 Liu W, Zhao S, Wang J, Shi J, Sun Y, Wang W, Ning G, Hong J, Liu R. Grape seed proanthocyanidin extract ameliorates inflammation and adiposity by modulating gut microbiota in high-fat diet mice. Mol Nutr Food Res 2017; 61(9):1601082. [ Links ]
44 Van Hul M, Geurts L, Plovier H, Druart C, Everard A, Ståhlman M, y col. Reduced obesity, diabetes, and steatosis upon cinnamon and grape pomace are associated with changes in gut microbiota and markers of gut barrier. Am J Physiol Endocrinol Metabol 2018; 314(4):E334-352. [ Links ]
45 Olmedilla-Alonso B, Jiménez-Colmenero F, Sánchez-Muniz FJ. Development and assessment of healthy properties of meat and meat products designed as functional foods. Meat Sci 2013;95(4):919-930. [ Links ]
46 Cofrades S, Benedí J, Garcimartin A, Sánchez-Muniz FJ, Jimenez-Colmenero F. A comprehensive approach to formulation of seaweed-enriched meat products: From technological development to assessment of healthy properties. Food Res Intern 2017; 99:1084-1094. [ Links ]
47 Jiménez-Colmenero F, Sánchez-Muniz FJ, Olmedilla-Alonso B. Design and development of meat-based functional foods with walnut: Technological, nutritional and health impact. Food Chem 2010; 123(4):959-967. [ Links ]
48 López-López I, Bastida S, Ruiz-Capillas C, Bravo L, Larrea MT, Sánchez-Muniz F, Cofrades S, Jiménez-Colmenero F. Composition and antioxidant capacity of low-salt meat emulsion model systems containing edible seaweeds. Meat Sci. 2009; 83(3):492498. [ Links ]
49 Schultz Moreira AR, Olivero-David R, Vázquez-Velasco M, González-Torres L, Benedí J, Bastida S, Sánchez-Muniz F.J. Protective effects of sea spaghetti-enriched restructured pork against dietary cholesterol: effects on arylesterase and lipoprotein profile and composition of growing rats. J Med Food 2014; 17(8):921-928. [ Links ]
Received: September 13, 2020; Accepted: September 20, 2020











 text in
text in