My SciELO
Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
The European Journal of Psychiatry
Print version ISSN 0213-6163
Eur. J. Psychiat. vol.30 n.1 Zaragoza Jan./Mar. 2016
Cerebrotendinous xanthomatosis with bradyphrenia and psychiatric disorders: a case with 18F-FDG PET imaging and a literature review
Shimeng Yua; Rong Dengb; Yang Tanc and Yunjian Zhangc
a Department of Neurology, Affiliated Hospital of Xinyang Vocational and Technical College, 411 Gongqu Road, Xinyang 464000. China
b Department of Neurology, Jingmen Hospital of Traditional Chinese Medicine, 15 Baimiao Road, Jingmen 448000. China
c Department of Neurology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, 1277 Jiefang Avenue, Wuhan 430022. China
ABSTRACT
Background and Objectives: Cerebrotendinous xanthomatosis (CTX) is a rare autosomal recessive lipid-storage disease caused by mutations in the CYP27A1. The purpose of this study is to determine the clinical characteristics, neuroimaging and mutation detect in a family with CTX systematically.
Methods: Collecting history materials and detecting the routine clinical biochemical tests and imaging examination, and for the first time taking the whole body positron emission tomography (PET)-CT examination for probed in the world to research abnormal metabolism activities in CTX. To observe the effect of treatment with chenodeoxycholic acid (CDCA) and stains before and after the intervention, using serum lipid level detection and neuropsychological evaluation. Genetic testing was carried out to screen the nine exons and exon-intron boundaries about 200-300bq of CYP27A1.
Results: A 37-year-old woman with typical clinical characteristics of CTX. Magnetic resonance imaging (MRI) of brain showed bilateral lesions in the dentate nucleus of the cerebellum, then, PET images revealed multiple abnormal hypermetabolism areas at distal tendon, and multifocal areas of hypometabolism in bilateral sides of cerebellar hemispheres, the frontal lobe and temporal lobe. Histopathology reveals accumulation of xanthoma cells and dispersed lipid crystal clefts in xanthomas. In genetic analysis, it shown an insertion of cytosine (77-78insC) located in the first exon of CYP27A1 in the proband.
Conclusions: We found that a Chinese patient presented a typical clinical feature of CTX along with clear correlation on both structural and functional imaging had a novel mutation in the CYP27A1 gene.
Key words: Cerebrotendinous xanthomatosis; Sterol 27-hydroxylase; CYP27A1.
Introduction
Cerebrotendinous xanthomatosis (CTX) is a rare autosomal recessive neurometabolic disease caused by mutation in CYP27A1 gene located on chromosome 2q33-qter, leading to increased deposition of cholesterol and cholestanol in multiple tissues, especially in the Achilles tendon and central nervous system1-3. Deficiency of sterol 27-hydroxylase leads to the reduced production of CDCA, subsequently resulted in the upregulation of cholesterol 7α-hydroxylase (CYP7A1). Moreover, upregulation of this rate-limiting enzyme in the classic bile acid pathway results in the elevated level of 7α-Hydoxy-4-cholesten-3-one, an efficient precursor to cholestanol4. There are no consensus data on the prevalence of CTX, the previous estimated incidence is 3-5/100,000 worldwide. CTX could present at any age and its prevalence may be much higher than previously recognized5. Bilateral Achilles tendon xanthomas, early-onset cataracts and neurological symptoms such as dementia and cerebellar ataxia are typical triad of CTX6. Imaging studies have a significant role in prompt diagnosis. MRI and cerebellar magnetic resonance spectroscopic (MRS) imaging demonstrated abnormal structural/metabolic changes in brains of the CTX patients7,8. Single photon emission computed tomography (SPECT) imaging of CTX cases showed presynaptic dopaminergic deficit associated with asymmetric parkinsonism symptom9,10. However PET analysis of CTX is rarely reported. Several hundred of confirmed cases and 50 different mutations of the CYP27A1 gene have been reported worldwide Since Van Bogaert reported the first patient in 193711. In this study, we describe the clinical characteristics, pathology, whole body PET-CT neuroimaging and one novel mutation of the CYP27A1 gene in a Chinese family with CTX.
Case presentation
History
A 37-year-old female presented with gait difficulty and psychiatric disorders was admitted to the hospital. Her history was obtained from her husband. When she was 17, bilateral Achilles tendon masses and progressively unsteady gait were found. Because it didn't significantly affect her life, she did not go to see a doctor. The Achilles tendon masses gradually increased in size over the years. Then, when she became 35 years old, she couldn't even do simple household works. She had slight bradyphrenia, spastic gait, mild dysarthria, psychiatric disorders such as emotional instability and lack of motivation. She often tried to hide some trivial items and kept seeking to ensure their safety. She had personality changes such as forced laughter or crying. She had poor appetite, easy fatigability, and pessimistic thinking. She was diagnosed as dysthymic disorder and received treatment with fluoxetine (40 mg/d) and olanzapine (5 mg/d), the antidepressant therapy played an effective role in her psychiatric manifestations but did not change intelligence and cognition evaluation. She received surgical excision of bilateral tendon swellings since the age of 37. Tendon xanthoma was diagnosed by needle biopsy of the swelling of Achilles tendon. After surgical excision of the bilateral tendon xanthomatosis, her shuffling gait alleviated without improvement of mental retardation and behavioral disorder. Her mother had bilateral cataract and eyelid xanthoma, and her son had a history of seizures. Her other family history was unremarkable.
Clinical and laboratory examinations
Neurologic examination revealed pes cavus, ankle clonus, bilateral deep tendon hyperreflexia and positive Babinski sign. Laboratory examination of blood uncovered hyperlipidaemia: Triglyceride was 5.11 mmol/L (< 5.11 mmol/L), cholesterol 6.54 mmol/L (3.2-5.2 mmol/L), high density lipoprotein cholesterol (HDL-C) 1.96 mmol/L (1.29-1.55 mmol/L), and low density lipoprotein cholesterol (LDL-C) 3.79 mmol/L (2.7-3.1 mmol/L), normal serum lactate 1.52 mmol/L (0.50-2.20 mmol/L). Her plasma cholestanol level was 2843 μg/dL (n.v 300-360μg/dL). Other routine blood tests (C-reactive protein, parathyroid hormone, thyroid function, rheumatoid immune markers and tumor markers) were unremarkable. Dual-energy X-ray absorptiometry of the posteroanterior lumbar spine (L1-L4), femoral neck and total hip uncovered decreased bone mineral density (BMD) (range 0.612-0.976 g/cm2), which revealed the existence of osteoporosis in the patient. Mild abnormal electroencephalography (diffuse slow waves in bilateral hemispheres) and somatosensory evoked potential (SEP) (prolonged latencies in N35, P45 and N60 waves) were detected. Electrophysiological conduction parameters of peripheral nerve were normal. Mini-mental state examination (MMSE) score = 20/30 and Montreal Cognitive Assessment (MoCA) Beijing Version score = 19/30. P300 latency period was 293ms. The mental status examination showed depressed and anxiety mood. An abdominal ultrasound revealed multiple cholecystic polyps; the biggest one was 7×6 mm.
MRI and PET/CT neuroimaging and histopathology
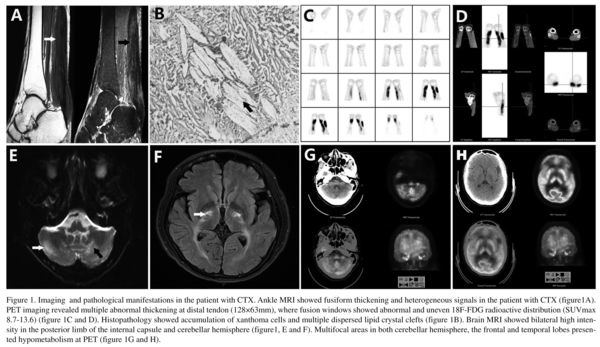
Magnetic resonance imaging (MRI) of bilateral ankle showed fusiform thickening and heterogeneous signals (Fig. 1A). Histopathology of the tendon mass showed an accumulation of xanthoma cells and multiple, dispersed lipid crystal clefts (Fig. 1B). Positron emission tomography (PET) with 18F-2-deoxy-2-fluoro-glucose (18F-FDG) revealed abnormally high radioactivity in Achilles tendons and adjacent regions (maximum standardized uptake values of 8.7-13.6) (Fig. 1C-D). Brain MRI revealed T2-weighted hyperintensities in the dentate nuclei, cerebellar hemispheres, and basal ganglion (Fig. 1E-F). PET also revealed hypometabolism in the cerebral lobes (especially the frontal and temporal lobe) and cerebellar hemisphere (Fig. 1G-H). The difference of brain metabolic changes between the CTX patient and normal people was remarkable (Supplemental materials).


Genetic analysis
Written informed consent was obtained from her family. Ethics committee in Union hospital approved this research protocol. Blood samples were obtained from the patient with CTX. Genomic DNA was extracted from peripheral blood leukocytes according to standard procedures. The sequence information of the CYP27A1 was obtained from NCBI database. CYP27A1 gene is located on chromosome 2, 43.62 kb in total. Nine exons of the CYP27A1 and intron adjacent regions about 200-300bq were amplified by polymerase chain reaction (PCR). The primers were designed with Primer 5. For example, exon1: Forward5' TCGCTCCGAACTGAC-TCCG 3', Reverse 5' GCAGCCTTCAC-TTT-CTGTCCAAC3'; exon2:5'GCCTCCAACATACACCTCAACG3', Reverse5'TTATC CACCTGCCTGCCCT3'; exon3-5: Forward5'CATAGAGGCTTATCTTTGTGCTGT3',Reverse5' AACTGGTTCAGGT TGGGAGC 3'; exon6-8: Forward5' CTCATACACCCTCCCATTACTG 3', Reverse5' ACACTCCTACCCTGTGCCTC 3'; exon9: Forward5'TACTCCTCGCAAGGG TGA3', Reverse5'CCTCAGATGCTGGGT AGTCA 3'. The DNA sequences were analyzed directly (Bio Miao Biological, Beijing, China). The genetic analysis showed an insertion of cytosine (c.-359_-358insC) located in the first exon of CYP27A1
Treatment and follow-up
Medications included olanzapine (5mg/d), atorvastatin (20mg/d), donepezil (5mg/d), vitamin D (800IU/d) and chenodeoxycholic acid (750mg/d). The blood lipid level of the patient and cholestanol concentration decreased after treatment for one month. MoCA score raised from 19 to 23. MMSE score increased from 20 to 22.
Discussion
Cerebrotendinous xanthomatosis (CTX) is a rare inherited lipid-storage disease, with an estimated prevalence of 3-5/100,000 people worldwide2, and is especially rare in Chinese population. Patients with inherited CTX lack sterol 27-hydroxylase (CYP27A1) and usually presents with diverse non-neurologic manifestations (ie, juvenile cataracts, chronic diarrhea, premature arteriosclerosis, osteoporosis, respiratory insufficiency and cardiac symptoms), and neurological dysfunction, such as dementia, cerebellar syndrome, epilepsy, pyramidal signs, peripheral neuropathy and myopathy6.
In this study, we described a patient with a confirmed diagnosis of CTX characterized by a typical manifestation with bilateral Achilles tendon xanthomas and neuropsychological symptoms. The patient in our study didn't have early non-neurologic dysfunction such as early-onset cataract, chronic diarrhea and neonatal cholestatic jaundice mentioned in the literature during her infancy12, but did have some symptoms such as osteoporosis, Achilles tendon xanthomas and cognitive decline. This case also showed some behavioral changes that have seldom been reported in the literature. CTX is a lipid metabolic disease with multi-system involvement, predominantly brain, tendon, lung and liver13. Kawabata et al. uncovered accumulation of foamy and giant cells with cholestanol in bronchoalveolar lavage fluids and lung biopsy of CTX patients, which demonstrated lungs were apparently involved in CTX14.
Psychiatric manifestations in the CTX are rare and non-specific, and often lead to significant diagnostic and treatment delay15. Lee et al. reported three siblings with CTX with psychiatric disorders such as long-term depressed mood, irritability, insomnia and pessimistic thinking. However, the effective treatments for physical manifestations of CTX did not have an effect on the IQ tests of the patients16, which is similar to our study. Early recognition of the psychiatric symptoms of CTX is important because both the psychiatric and neurological symptoms respond to treatment with CDCA17.
Elevated plasma 5 alpha-cholestanol concentration detected by gas chromatography-mass spectrometry (GC-MS) is a biomarker in CTX patients18. Plasma cholestanol levels along with SEP assessment could support a sensitive index of improved biochemical and neurological function before and after drug treatment. It can also be used to distinguish CTX from other lipid-storage disorders like sitosterolemia and familial hypercholesterolemia which share similar clinical features such as xanthomas and cardiac disease19. However a retrospective review involving twenty-five patients revealed that cholestanol levels didn't correlate with clinical presentation, severity or response to therapy20.
MRI revealed involvement of the dentate nucleus, adjacent cerebellar and periventricular white matter hyperintensities6. Gray matter and whiter matter volume correlated closely with neuropsychological results are diffusely decreased in CTX patients21. Several previous MR spectroscopic study disclosed increased lactate and lipid peaks, and diffuse decreased N-acetylaspartate (NAA) peaks in the FLAIR-hypointense lesion7,22. SPECT/CT imaging revealed regional cerebral blood flow (rCBF) changes in multiple brain lobes before and after therapy, which might be an useful tool to follow the therapy response in CTX patients23. SPECT imaging using special photographic developer (eg, 99mTc-sestamibi, 123I -FP-CIT) could assess the mitochondrial status and presynaptic dopaminergic function of CTX9,24. However the whole body PET analysis of CTX is rarely reported. 18F-FDG PET/CT imaging in our study revealed multiple abnormal meta-bolism areas at quadriceps tendons and Achilles tendons, multifocal areas of hypometabolism in bilateral cerebellar hemisphere, the frontal lobe and temporal lobe. The brain metabolism changes were correlating well with the patient's clinical status (cognitive decline, behavior changes, cerebellar symptoms and pyramidal signs).
The CYP27A1 gene had been firstly repor-ted to be associated with CTX Since 1991(1). Various mutation types included deletion (14%), insertion (2%), splice site mutation (18%), missense (approximately 45%) and nonsense mutations (approximately 20%) in all nine exons of CYP27A1 have been detec-ted11. 50% of mutations in CYP27A1 were detected in the region of exons 6-8, 16% in exon 2, and 14% in exon 425. Mutation in exon 1 is rare. An insertion of cytosine loca-ted in the section start of the first exon of CYP27A1 (77-78insC) was detected in our study. It is important to note that none of the CYP27A1 gene mutations could be associated with any special symptom, clinical feature, onset age or prognosis20.
The mean age of onset in the living CTX patients is 19, an average age on diagnosis is 35 years (range 23-44) and a diagnostic delay of 16 years (range 2-34)20. In our study, the patient was diagnosed with CTX at the age of 37 years. Early detection and diagnosis of CTX is crucial, for early and long-term treatment of CTX with CDCA (750mg/d) and it could improve neurological symptoms and even reverse the progression of CTX6. The potential mechanisms maybe that CDCA given exogenously inhibits the bile acid synthesis by negative feed back and further prevents accumulation of cholestanol in tissues by normalizing cholestanol concentration26. Unfortunately, we conjugate ursodeoxycholic acid (UDCA) therapy with symptomatic treatment to cure the patient because CDCA isn't available in china. Fortunately, the CTX patient didn't exacerbate in the follow-up with the above medication. We also revealed that early detection and intervention may correct the abnormal lipid metabolism and possibly arrest the progression of CTX.
There are so many clinical researches about CTX, but the basic animal research about the pathogenesis of CTX is still weak. It is interesting that the sterol 27-hydroxylase gene knock out doesn't lead to formation of xanthomas in tendons and brain of the knock out mice27. Although some accumulations of cholestanol were detected in female mice, this accumulation is unremarkable and is not accompanied by accumulation of cholesterol. The specific mechanism for the difference between CTX patients and the mouse model is unclear. The relationship between the deposition of cholestanol and the development of xanthomas deserves further study and a suitable animal model for cerebrotendinous xanthomatosis is still lacking.
Conclusions
We found that a Chinese patient presented a typical clinical feature of CTX along with clear correlation on both structural and functional imaging had a novel mutation in the CYP27A1 gene. It is firstly whole body 18F-FDG PET/CT imaging report about CTX in the world.
Acknowledgements
The authors want to express their gratitude to the patients and her families for participating in this research.
Consent
Written informed consent was obtained from the patient or their relative for publication of study.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
Shimeng Yu wrote the paper and revised the manuscript. Rong Deng collected the blood and tissue samples for study. Yang Tan performed the genetic analysis. Yunjian Zhang designed the experiment and final approval of the revision. All authors have read and approved the final manuscript.
References
1. Cali JJ, Hsieh CL, Francke U, Russell DW. Mutations in the bile acid biosynthetic enzyme sterol 27-hydroxylase underlie cerebrotendinous xanthomatosis. J Biol Chem. 1991; 266(12): 7779-83. [ Links ]
2. Moghadasian MH, Salen G, Frohlich JJ, Scudamore CH. Cerebrotendinous xanthomatosis: a rare disease with diverse manifestations. Arch Neurol. 2002; 59(4): 527-9. [ Links ]
3. Tian D, Zhang Z-Q. 2 Novel deletions of the sterol 27-hydroxylase gene in a Chinese Family with Cerebrotendinous Xanthomatosis. BMC Neurol. 2011; 11: 130. [ Links ]
4. Björkhem I, Hansson M. Cerebrotendinous xanthomatosis: an inborn error in bile acid synthesis with defined mutations but still a challenge. Biochem Biophys Res Commun. 2010; 396(1): 46-9. [ Links ]
5. Lorincz MT, Rainier S, Thomas D, Fink JK. Cerebrotendinous xanthomatosis: possible higher prevalence than previously recognized. Arch Neurol. 2005; 62(9): 1459-63. [ Links ]
6. Nie S, Chen G, Cao X, Zhang Y. Cerebrotendinous xanthomatosis: a comprehensive review of pathogenesis, cli-nical manifestations, diagnosis, and management. Orphanet J Rare Dis. 2014; 9: 179. [ Links ]
7. Embiruçu EK, Otaduy MC, Taneja AK, Leite CC, Kok F, Lucato LT. MR spectroscopy detects lipid peaks in cerebrotendinous xanthomatosis. AJNR Am J Neuroradiol. 2010; 31(7): 1347-9. [ Links ]
8. Chang CC, Lui CC, Wang JJ, Huang SH, Lu CH, Chen Cm, et al. Multi-parametric neuroimaging evaluation of cerebrotendinous xanthomatosis and its correlation with neuropsychological presentations. BMC Neurol. 2010; 10: 59. [ Links ]
9. Schotsmans K, De Cauwer H, Baets J, Ceyssens S, van den Hauwe L, Deconinck T, et al. Cerebrotendinous xanthomatosis presenting with asymmetric parkinsonism: a case with I-123-FP-CIT SPECT imaging. Acta Neurol Belg. 2012; 112(3): 287-9. [ Links ]
10. Lagarde J, Roze E, Apartis E, Pothalil D, Sedel F, Couvert P, Vidailhet M, Degos B. Myoclonus and dystonia in cerebrotendinous xanthomatosis. Mov Disord.2012; 27(14): 1805-10. [ Links ]
11. Gallus GN, Dotti MT, Federico A. Clinical and molecular diagnosis of cerebrotendinous xanthomatosis with a review of the mutations in the CYP27A1gene. Neurol Sci. 2006; 27(2): 143-9. [ Links ]
12. Verrips A, van Engelen BG, Wevers RA, van Geel BM, Cruysberg JR, van den Heuvel LP, Keyser A, Gabreëls FJ. Presence of diarrhea and absence of tendon xanthomas in patients with cerebrotendinous xanthomatosis. Arch Neurol. 2000; 57(4): 520-4. [ Links ]
13. Bhattacharyya AK, Connor WE. Beta-sitosterolemia and xanthomatosis. A newly described lipid storage disease in two sisters. J Clin Invest. 1974; 53(4): 1033-43. [ Links ]
14. Kawabata M, Kuriyama M, Mori S, Sakashita I, Osame M. Pulmonary manifestations in cerebrotendinous xanthomatosis. Intern Med. 1998; 37(11): 922-6. [ Links ]
15. Fraidakis MJ. Psychiatric manifestations in cerebrotendinous xanthomatosis. Transl Psychiatry. 2013; 3: e302. doi: 10.1038/tp.2013.76. [ Links ]
16. Lee Y, Lin PY, Chiu NM, Chang WN, Wen JK. Cerebrotendinous xanthomatosis with psychiatric disorders: report of three siblings and literature review. Chang Gung Med J. 2002; 25(5): 334-40. [ Links ]
17. Berginer VM, Foster NL, Sadowsky M, Townsend JA 3rd, Siegel GJ, Salen G. Psychiatric disorders in patients with cerebrotendinous xanthomatosis. Am J Psychiatry. 1988; 145(3): 354-7. [ Links ]
18. De Barber AE, Luo J, Giugliani R, Souza CF, Chiang JP, Merkens LS, Pappu AS, Steiner RD. A useful multi-analyte blood test for cerebrotendinous xanthomatosis. Cli Bio-chem. 2014; 47(9): 860-3. [ Links ]
19. Chalès G, Coiffier G, Guggenbuhl P. Miscellaneous non-inflammatory musculoskeletal conditions. Rare thesaurismosis and xanthomatosis. Best Pract Res Clin Rheumatol. 2011; 25(5): 683-701. [ Links ]
20. Pilo-de-la-Fuente B, Jimenez-Escrig A, Lorenzo JR, Pardo J, Arias M, Ares-Luque A, et al. Cerebrotendinous xanthomatosis in Spain: clinical, prognostic, and genetic survey. Eur J Neurol. 2011; 18(10): 1203-11. [ Links ]
21. Guerrera S, Stromillo ML, Mignarri A, Battaglini M, Marino S, Di Perri C, et al. Clinical relevance of brain volume changes in patients with cerebrotendinous xanthomatosis. J Neurol Neurosurg Psychiatry. 2010; 81(11): 1189-93. [ Links ]
22. Mignarri A, Dotti MT, Del Puppo M, Gallus GN, Giorgio A, Cerase A, et al. Cerebrotendinous xanthomatosis with progressive cerebellar vacuolation: six-year MRI follow-up. Neuroradiology. 2012; 54(6): 649-51. [ Links ]
23. Caroppo P, D'Agata F, Mignarri A, Stromillo ML, Dotti MT, Mongini T. Brain metabolism changes after therapy with chenodeoxycholic acid in a case of cerebrotendinous xanthomatosis. Neurol Sci. 2013; 34(9): 1693-6. [ Links ]
24. Chen SF, Chang CC, Huang SH, Lu CH, Chuang YC, Pan TL, et al. 99mTc-sestamibi thigh SPECT/CT imaging for assessment of myopathy in cerebrotendinous xanthomatosis with histopathological and immunohistochemical correlation. Clin Nucl Med. 2014; 39(3): e202-7. [ Links ]
25. Lorbek G, Lewinska M, Rozman D. Cytochrome P450s in the synthesis of cholesterol and bile acids-from mouse models to human diseases. FEBS J. 2012; 279(9): 1516-33. [ Links ]
26. Panzenboeck U, Andersson U, Hansson M, Sattler W, Meaney S, Björkhem I. On the mechanism of cerebral accumulation of cholestanol in patients with cerebrotendinous xanthomatosis. J Lipid Res. 2007; 48(5): 1167-74. [ Links ]
27. Rosen H, Reshef A, Maeda N, Lippoldt A, Shpizen S, Triger L, et al. Markedly reduced bile acid synthesis but maintained levels of cholesterol and vitamin D metabolites in mice with disrupted sterol 27-hydroxylase gene. J Biol Chem. 1998; 273(24): 14805-12. [ Links ]
![]() Correspondence:
Correspondence:
Yunjian Zhang
Department of Neurology
Union Hospital, Tongji Medical Collage
Huazhong University of Science and Technology. China
E-mail: zhangyunjian66@126.com
Rong Deng
Department of Neurology
Jingmen Hospital of Traditional Chinese Medicine
15 Baimiao Road, Jingmen 448000. China
E-mail: 37701640@qq.com
Received: 16 June 2015
Revised: 15 October 2015
Accepted: 24 November 2015