The importance of this work lies in the importance of the emotional experience for survival (Darwin, 1872; LeDoux, 2012) and for the adequate psychosocial development of human beings (Borges & Naugle, 2017; Pleeging et al., 2019). When the emotional experience is deficient or when it is not adequate to the situational demand, it becomes a problem for adaptation (Aldao et al., 2010; Colombo et al., 2020). The emotional experience takes place in the very organism of the person who experiences it (Blair & Diamond, 2008; Raz et al., 2016). For this reason, we consider that, together with the knowledge of the characteristics of the process of emotional experience, it is essential to understand its biological foundations in order to facilitate resources for the improvement of the emotional state based on its foundations.
The Emotional Experience
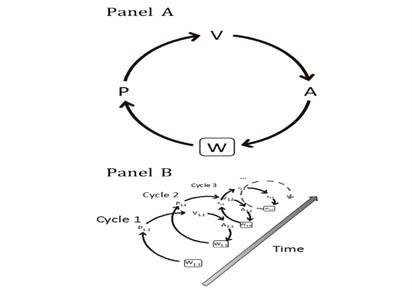
The emotional experience is the dynamic result of the interaction between the emotional reaction and emotional regulation (Ochsner et al., 2012; Ochsner & Gross, 2014). In the Process Model of Emotion Regulation, hereafter PMER- (Gross, 2015) and based on the World-Perception-Perception-Valuation-Action (hereafter, W-PVA) framework of Ochsner & Gross (2014), the activation of the emotional experience occurs in a certain world (W) with the activation of perception (P). In this initial perception stage, sensory inputs are encoded for their valuation to take place (V). Valuations are made by an overlapping set of brain systems. And three types of valuations are differentiated located on the cognitive processing continuum. At the most basic level, there are basic valuations which represent relatively direct associations between perceptions and the valence given to them. At the intermediate level, contextual valuations refer to evaluations about stimulus-response relationships that are made based on three categories of information: the subject's history, the social context, and the subject's motivations. Finally, at the most complex cognitive level, there are conceptual valuations, which represent abstract valuations about different stimuli and realities. And at the level of activated valuation, emotion is generated. In turn, on any level of valuation (V) the action (A) is activated, which can be either mental (for example, a memory) or belonging to the organism (for example, an increase in heart rate). The actions generated have consequences on the surrounding reality, on the world (W). In turn, the changes generated in the world (W), will again affect the P, and this again affects the V, which will also affect the A again. Emotional experience occurs longitudinally over time on the basis of the aforementioned interdependencies. And the W-PVA cycle will cease when the emotional experience ends (Figure 1).
Also in PMER theory (Gross, 2015), the emotional experience is defined as the processual result of the interaction between the emotional reaction and emotional regulation. The emotional reaction is automatic (Behnke et al., 2022), whereas emotional regulation is conscious and refers to the efforts we subjects make to change our own emotions (Gross & Thompson, 2007). Adequate emotional regulation allows us to regulate our own emotional reactions in order to also be able to manage our own discomfort (Goldin et al., 2019; Uccula et al., 2020). In PMER theory, emotional regulation is composed of three stages: identification, selection, and implementation. Identification corresponds to the stage in which the subject who is experiencing the emotional reaction decides whether to modify the reaction or not. In relation to the W-PVA cycle, the perception phase (P) corresponds to detecting the experience of emotion; the valuation phase (V) corresponds to evaluating whether the emotional reaction being experienced is sufficiently positive or negative to activate regulation; and the regulation itself occurs in the action phase (A).
Biological Foundations of Emotional Experience
Based on neuroimaging findings, Etkin et al. (2015) have distinguished different neuroanatomical areas related to the processes of emotional response and emotional regulation. Thus, in relation to experiences of emotional reaction, the areas involved are as follows: on the one hand, the subcortical system composed of the amygdala, the ventral striatum, and the periaqueductal gray matter; and, on the other hand, a set of cortical regions including the anterior insula and the anterior dorsal cingulate (Beissner, et al., 2013; Costafreda et al., 2008; Etkin et al., 2015). The variety of information encoding that occurs in these structures explains, in part, the cognitive, subjective, motor, and physiological multidimensionality of emotional experience. Each structure processes information at different levels. For example, the central regions of the limbic system—such as the amygdala, ventral striatum, and periaqueductal gray matter—process simple motivational features of a stimulus, such as the threat we feel from a large spider; and the cortical regions, such as the insula, provide additional interoceptive information.
From an evolutionary point of view, the brain areas characteristic of prototypical adult emotional experience are the result of evolutionary development (Decety et al., 2011; Diamond, 2002; Michalska et al., 2013; Thomas et al., 2017). In such development the human brain presents a prolonged heterogeneous maturation, whose development follows a rostral caudal direction, a prior development from phylogenetically older structures towards more recent ones directed from areas of low neuronal expansion towards those of high expansion. Thus, certain areas of the cerebral cortex, being characteristic of the areas of long expansion and phylogenetically more recent, are the ones that present a more prolonged development as occurs with the prefrontal cortex (Aubert-Broche et al., 2013) whose interneurons are the last neurons to mature (Lagercrantz, 2016). Thus, from an evolutionary perspective, the early postnatal years are an exceptionally dynamic and critical period of structural, functional, and connectivity development of the human brain (Haartsen et al., 2016; Li et al., 2019). Over approximately the first two decades, the process of synaptogenesis will widen the cortical columns and through the process of myelination the processing speed of multiple brain areas will be enhanced (Dehaene-Lambertz & Spelke, 2015). It has also been observed how the amygdala, together with the posterior part of the insular cortex, exerts a strong influence on emotional processing in childhood, the consequence of which is that children tend to experience emotional reactions more intensely than in adulthood (Casey et al., 2005; Silvers et al., 2016, 2017).
Vagal Tone: an Indicator of Emotional Experience
Having differentiated emotional reaction and emotional regulation both as autonomous (and interdependent) processes and as processes located in different brain areas which develop throughout the evolutionary development, we will now focus on the vagal tone indicator.
Porges (1992) defined cardiac vagal tone as a physiological measure of stress, and he equated high vagal tone with high heart rate variability (hereafter HRV) and an experience of homeostasis with positive emotional valence; conversely, low vagal tone implies low HRV and a stressful experience with negative emotional valence (Porges, 2022). HRV is also defined as the average time between heartbeats (Task Force, 1996).
In polyvagal theory (Porges, 2007, 2009) it is considered that the origin of emotional reactions is found in the automatic and non-conscious perception that the autonomic nervous system performs based on perceived safety, risk, or extreme risk. Based on this theory, the autonomic nervous system is hierarchically organized on the configuration of the vagus nerve. The vagus nerve has multiple innervations and connections to much of the organism (Berthoud & Neuhuber, 2000; Neuhuber & Berthoud, 2021). And structurally the vagus nerve is composed of the ventro-vagal branch and the dorso-vagal branch; the dorso-vagal branch has no myelin and is phylogenetically the oldest; while the ventro-vagal branch has myelin and is phylogenetically the most recent (Gourine et al., 2016, Porges, 1995). Both complexes together with the sympathetic-adrenal axis, characteristic of the sympathetic nervous system, make up the three neurobiological circuits of the autonomic nervous system (Porges, 2022).
The ventro-vagal complex (VVC) or myelinated vagus is activated when the organism perceives security. Its center is located in the nucleus ambiguus (NA) and its innervations are directed to supradiaphragmatic areas. From the ventral area of the NA, it exchanges information with the nucleus of the solitary tract (NST), with some cranial nerves and with the sinoatrial node of the heart. The NST establishes connections with the hypothalamus, the limbic system, the periaqueductal gray matter, the amygdala, and different parts of the cortex (Berthoud & Neuhuber, 2000). In relation to the cranial nerves in the NA there are also several innervations of the glossopharyngeal nerve and the facial nerve. As a result of the automatic activation of the ventro-vagal complex, the face and voice show prosocial patterns such as, for example, a smile and a pleasant tone of voice (Porges, 2004). And in relation to the sinoatrial node, at the cardiac level the myelinated vagus is an inhibitor of the sympathetic system, which functions as a brake enabling a rapid slowing of the heart rate and an increase in HRV (Porges, 1995). Thus, the emotional reaction generated in the organism is characteristic of a state of well-being.
When the organism automatically detects risk, the influence of the ventral vagus disappears, and the sympathetic-adrenal system is activated. The sympathetic-adrenal system is part of the sympathetic nervous system and is considered an adaptive mobilization system that supports fight-flight behaviors; this, together with the perception of risk, is associated with a withdrawal of the parasympathetic influence of the ventro-vagal complex (Porges, 2004, 2022). Thus, when the organism processes risk, the parasympathetic influence of the heart is deactivated, and the sympathetic-adrenal system is activated. The sympathetic influence on heart rate is mediated by the release of epinephrine and norepinephrine (Kim et al., 2018). The beta-adrenergic receptors are activated upon the release of these hormones resulting in cAMP-mediated membrane protein phosphorylation (Brown et al., 1979). Thus, in the absence of the influence of the ventral vagus on the sinoatrial node and as a consequence of activation of the sympathetic-adrenal system, heart rate increases and HRV decreases. And although the blood-brain barrier prevents epinephrine from acting on cognitive functions (Weil-Malherbe, 1959), beta-adrenergic receptors in the vagus nerve allow the reuptake of norepinephrine in the brain (Chen & Williams, 2012; Noble et al., 2019), supporting the experience of stress more solidly and thus the functioning of cognitive functions is subordinated to amygdalar functioning (Arnsten et al., 2015). The emotional reaction generated is typical of a state of emotional distress.
Finally, when extreme risk is detected, the dorsal-vagal complex (DVC) or unmyelinated vagus is activated, which mainly innervates subdiaphragmatic areas. The neurobehavioral functions of this complex are immobilization or passive adaptations that include apparent death and loss of consciousness (Porges, 2007). Thus, the emotional reaction generated will be related to the characteristics of emotional shock.
The validity of the heart rate and HRV indicator is based on the fact that ventral vagal efferents are cardioinhibitory and synapse in the sinoatrial node of the heart (Goggins et al., 2022). When myelinated ventral vagal fibers of the NA are activated through their parasympathetic influence on the sinoatrial node, they reduce heart rate by increasing the time between heartbeats, increasing the HRV as an indicator of high vagal tone. However, when risk is detected, the parasympathetic influence of the vagus ventral to the sinoatrial node disappears, producing an increase in heart rate, a reduction in vagal tone and HRV. The activation of the sympathetic-adrenal complex generates stressful emotional experiences characteristic of low vagal tone (Porges, 1995, 2022). This is why HRV is also considered a biomarker of stress with which it maintains an inverse relationship: the higher the HRV level the lower the stress, and the lower the HRV the more the stress (Balzarotti et al., 2017; Goessl et al., 2017).
Regarding the experience of the emotional reaction and its evolutionary development, given that our starting assumption is that the genesis of every emotional reaction is related to the experiences of security, risk, and extreme risk (Porges, 1995, 2022), we should mention that from the moment of birth the human being possesses the adequate functionality of the neurobiological circuits of the ventro-vagal complex, the sympathetic-adrenal axis, and the dorsal-vagal complex. However, given that the brain is immersed in its evolutionary development process, the different brain areas mentioned in the postulates of polyvagal theory do not possess throughout childhood and adolescence the sufficient maturity required for their adequate performance. Nevertheless, the connection from the ambiguous nucleus of the vagus nerve to the sinoatrial node of the heart is fully developed, so we can consider that the validity of the HRV is justified. Thus, throughout the ontogenetic development, with the exception of pathological states, a reduction in HRV will imply the experience of emotional realities characteristic of the fight-flight system of the sympathetic autonomic nervous system, while an increase in HRV will make possible the experience of emotional realities close to the state of well-being and security characteristic of the activation of the myelinated vagus nuclei of the NA (Porges, 2022).
Based on this knowledge, two types of interventions aimed at increasing HRV in order to provide emotional well-being will be described below.
Interventions to Increase Vagal Tone
Biofeedback is a widely used method to train and educate people in the skills of voluntary control of some physiological functions, such as breathing, which consists of providing users with instantaneous information on the variations that occur in their own physiological activity (Schwartz & Andrasik, 2003).
Thus, through HRV biofeedback programs and by practicing relaxed breathing subjects learn to breathe in a way that increases HRV (Kiselev et al., 2016). In this regard, it has been observed that a breathing-focused HRV biofeedback teaches people to breathe at a rate of approximately six breaths per minute (Karavaev et al., 2013).
HRV biofeedback can be performed by fitting a person with a device that connects to a computer and provides real-time feedback on their HRV. By observing the impact of breathing on HRV in real time, they learn to breathe—through trial and error and feedback—thus improving their HRV values.
Various HRV biofeedback interventions focused on breathing have increased the values of participating HRV subjects in both adult (Aritzeta et al., 2017; Goessl et al., 2017; Lantyerm et al.,2013) and child populations (Aranberri Ruiz, et al., 2022; Aritzeta et al., 2022; Jones et al., 2019; Rush et al., 2017).
As an emerging neuromodulation therapy, transcutaneous auricular vagus nerve stimulation (hereafter taVNS) has been shown to be safe and effective for major depressive disorders, insomnia, and anxiety (Wang et al., 2022). This procedure is now CE marked (European Community Conformity Mark) for depression and anxiety (Farmer et al., 2020). And in January 2022 the United States of Food and Drug Administration (FDA) granted ElectroCore's noninvasive vagus nerve stimulator (nVNS) designation as an innovative device for treating post-traumatic stress disorder (PTSD).
The mechanism of action of this procedure is as follows: the external ear is the only site to which the vagus nerve sends its peripheral branch, the auricular vagus nerve (Trevizol et al., 2015; Goggins et al., 2022). From the auricular pathway of the nerve the fibers project to the nucleus of the solitary tract (NST) (Farmer et al., 2020). Neuronal anatomy has shown that the auricular branch of the vagus nerve projects to the NST which in turn is connected to other brain regions, such as the locus coeruleus, parabrachial nucleus, hypothalamus, thalamus, amygdala, hippocampus, anterior cingulate cortex, anterior insula, and lateral prefrontal cortex (Beekwilder & Beems, 2010). The NST is the source nucleus for all vagal afferents and its stimulation affects both lower motor neurons in the brainstem and upper motor neurons in the cerebral cortex (Komisaruk et al., 2022; Porges, 2007). Within the medulla, the NST projects directly to the dorsal motor nucleus (DMN) of the vagus nerve and to the nucleus ambiguus (NA), from where preganglionic parasympathetic efferents to visceral organs originate (Frangos et al., 2015). In turn, from the NST, signals will be sent to the ventrolateral caudal nuclei of the medulla, which will send information to the ventrolateral rostral nuclei, which through the intermediolateral cell columns reduce sympathetic influence (Butt et al., 2020). Thus, through parasympathetic influence on the heart with taVNS, HR will be reduced and HRV will be increased.
Although in one intervention the relationship between HRV and taVNS has been questioned (Wolf et al., 2021), in different investigations a robust relationship between taVNS and increased HRV is observed (Antonino et al., 2017; Bretherton et al., 2019; Clancy et al., 2014; De Couck et al., 2017; Sclocco et al, 2019).
Conclusions
Both biofeedback interventions of HRV focused on breathing, as well as the taVNS through different mechanisms of action generate an increase in HRV in the organism, which implies an increase in vagal tone, typical of emotional states with positive valence related to states of security that generate experiences of emotional well-being (Porges, 2022).
The therapeutic potential of the two interventions is justified both by the knowledge of the biological bases addressed throughout this work, as well as by the understanding of the PMER Theory and W-PVA cycle mentioned above. By means of both procedures, actions (A) are generated in the organism that make possible an increase in HRV, generating in turn a safer world (W) where emotional experiences have greater emotional wellbeing.
On the one hand, by means of biofeedback of HRV, the subjects, infants (for the aforementioned reasons of evolutionary development, mainly from 7 years of age), adolescents, and adults learn to breathe at a rate of approximately 6 breaths per minute in such a way that this learning makes it possible to improve their emotional well-being. Thus, after the intervention, the subjects are able to breathe in a way that increases their own HRV and their own psychological well-being (Aranberri Ruiz et al., 2022; Aritzeta et al., 2022). Therefore, when such subjects are immersed in an emotional reality of low HRV, typical of states of emotional discomfort, after identifying their own state of emotional discomfort, they will be able to select and implement the learned breathing pattern automatically to increase their own HRV values and thus approach a state of emotional well-being.
On the other hand, through taVNS, even though no emotional self-regulation procedure is taught to the subject, the professional trained in the use of taVNS provides the intervening subject with an increase in HRV, making a better emotional state possible. That is, this procedure, like the biofeedback mentioned above, affects the subject's world (W) through the actions (A) of increased vagal tone carried out, this time not by the subject him- or herself, but by the trained professional.