Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Nutrición Hospitalaria
versión On-line ISSN 1699-5198versión impresa ISSN 0212-1611
Nutr. Hosp. vol.27 no.6 Madrid nov./dic. 2012
https://dx.doi.org/10.3305/nh.2012.27.6.6010
Antitumor effect of oleic acid; mechanisms of action. A review
Efecto antitumoral del ácido oleico; mecanismos de acción; revisión científica
C. Carrillo, M.a del M. Cavia and S. R. Alonso-Torre
Área de Nutrición y Bromatología. Facultad de Ciencias. Universidad de Burgos. Burgos. Spain.
ABSTRACT
Introduction: The beneficial effects of oleic acid in cancer processes can no longer be doubted, but little is known about the mechanisms of action behind this phenomenon.
Aim: The aim of the present review is to clarify whether oleic acid has an effect on important mechanisms related to the carcinogenic processes.
Methods: We searched electronic databases and bibliographies of selected articles were inspected for further reference. We focused our research on two cellular transformations characterizing cancer development: proliferation and cell death or apoptosis.
Results: Numerous studies have reported an inhibition in cell proliferation induced by oleic acid in different tumor cell lines. Herein, oleic acid could suppress the over-expression of HER2 (erbB-2), a well-characterized oncogene which plays a key role in the etiology, invasive progression and metastasis in several human cancers. In addition, oleic acid could play a role in intracellular calcium signaling pathways linked to the proliferation event. Regarding cell death, oleic acid has been shown to induce apoptosis in carcinoma cells. The mechanisms behind the apoptotic event induced by oleic acid could be related to an increase in intracellular ROS production or caspase 3 activity. Several unsaturated fatty acids have been reported to induce apoptosis through a release of calcium from intracellular stores. However, evidence regarding such a role in oleic acid is lacking.
Conclusions: Oleic acid plays a role in the activation of different intracellular pathways involved in carcinoma cell development. Such a role could be the root of its antitumoral effects reported in clinical studies.
Key words: Oleic acid. Apoptosis. Proliferation. Intracellular signaling.
RESUMEN
Introducción: Los estudios epidemiológicos atribuyen un papel protector al ácido oleico frente a determinados tipos de cáncer. Sin embargo, el conocimiento relativo al mecanismo por el cual tal ácido graso ejerce sus efectos es escaso.
Objetivo: La presente revisión bibliográfica tiene como objetivo recopilar aquellos trabajos que centran su atención en los mecanismos intracelulares que podrían explicar los efectos clínicos atribuidos al ácido oleico.
Métodos: Se ha realizado una búsqueda bibliográfica a través de bases de datos electrónicas y las referencias de los artículos de interés han sido utilizadas como fuente de búsquedas más avanzadas. Nuestra revisión se ha centrado en la descripción de dos de las transformaciones celulares que caracterizan el desarrollo de cáncer: proliferación y muerte celular.
Resultados: Numerosos estudios atribuyen un papel inhibidor de la proliferación de células tumorales al ácido oleico. Entre los mecanismos de acción, se encuentran su capacidad para suprimir la expresión de HER2 (erbB-2), un oncogén bien conocido por su implicación en la etiología, progresión y metástasis de distintos tipos de cáncer. Además, el ácido oleico podría jugar un papel en la activación de la señalización de calcio intracelular, rutas igualmente ligadas a la proliferación celular. En cuanto a su papel en los fenómenos de muerte celular, el ácido oleico puede inducir apoptosis en células tumorales describiéndose como mecanismos implicados la producción de intracelular de especies reactivas o la activación de la actividad caspasa 3. Aunque muchos estudios relacionan la apoptosis inducida por los ácidos grasos insaturados con la liberación de calcio de los depósitos intracelulares, faltan estudios que aclaren el papel del ácido oleico a este respecto.
Conclusión: El ácido oleico juega un papel en la activación de diferentes rutas intracelulares implicadas en el desarrollo de células tumorales. Estos mecanismos podrían ser la base de los efectos protectores que le atribuyen los estudios clínicos.
Palabras clave: Ácido oleico. Apoptosis. Proliferación celular. Señalización celular.
Abbreviations
OA: Oleic acid.
PUFA: Polyunsaturated fatty acids.
MUFA: Monounsaturated fatty acids.
SOC: Store-operated channels.
SOCE: Store-operated Ca2+ entry.
ROS: Reactive oxygen species.
DHA: Docosahexaenoic acid.
Introduction
General evidence of the antitumor effect of oleic acid
Epidemiological studies have suggested a positive association between the total fat intake and the risk of cancer, particularly breast, colorectal and prostate cancers.1 Carcinogenesis models have also provided evidence for a lipid specific action beyond their caloric supply, thus suggesting that the type of fat and its unique composition are of greater importance than overall fat intake.2-7
Whereas a high intake of n-6 polyunsaturated fatty acids (PUFA) has tumor-enhancing effects, n-3 PUFA have inhibitory effects. However, there are few experimental studies addressing the role of monounsaturated fatty acids (MUFA) of the n-9 family, such as oleic acid (OA), on cancer, if compared to the investigations about the role of other dietary lipids.
OA has attracted much attention, especially in the last few years, as the "Mediterranean diet", characterized by a high olive oil (rich in OA) consumption, has been traditionally linked to a protective effect against cancer.8 A wide range of studies have been conducted into breast cancer, where a potential protective effect of olive oil and OA has been described.9-11 In addition, epidemiological studies suggest that olive oil may have a protective effect on colorectal cancer development.12-14 In this sense, some animal studies have also shown that dietary olive oil prevented the development of colon carcinomas in rats, corroborating that olive oil may have chemopreventive properties against colon carcinogenesis.15-17
Finally, a novel approach to chemotherapy has the potential to yield novel dietary-drug combinations that can provide additive or even synergistic protection against the progression of cancer and it is especially relevant when the etiology of disease development has varied mechanistic routes. With regard to this novel approach, OA has been reported to act synergistically with cytotoxic drugs, thus enhancing their antitumor effect.17-19
Thus, both epidemiological and animal studies have reported a protective role of oleic acid in several cancers. However, the mechanisms behind the antitumor effect of such a fatty acid are not well understood. The aim of the present review is to clarify where the knowledge concerning this topic is.
Materials and methods
Search strategy
We consulted studies published in electronic databases such as Pubmed or Medline. The bibliographies of selected articles were inspected for any further reference.
Firstly, we studied the title and abstract of all kind of papers (regular or review papers) with a potential interest to understand the role of oleic acid in cancer events. We mostly focused on those related to the mechanisms of action of such a fatty acid at the cellular level. Thus, the main key words used in the search were: "oleic acid", "apoptosis", "proliferation", "carcinoma cells", "intracellular signaling". Then, the text of the main trials that met the criteria previously mentioned was fully examined to extract the specific data included in the review.
Results and discussion
Cancer development is characterized by specific cellular transformations involving changes in proliferation rates, inactivation of tumor-suppressor genes and inhibition of apoptosis. Thus, we will describe the ability of oleic acid to induce apoptosis and/or inhibit cell proliferation in cancer cell lines.
Oleic acid-proliferation
It has been recently shown that OA promoted the growth of non-malignant cells but, in fact, it had the opposite effect, in malignant cells.20 Several researchers have also demonstrated that both oleic and α-linolenic acid showed a proliferation inhibition effect on prostate carcinoma cells.21,22 According to these results, numerous studies have also reported an inhibition in cell proliferation induced by OA in different tumor cell lines.23,24 However, other inconsistent results have been also obtained, including non-promoting, weak-promoting, and even promoting effects on tumor growth.25-29 Such contradictory observations may be in part the result of the different methods used in the determinations.
On the basis that OA and other fatty acids are good cellular fuels that can be degraded through β-oxidation when imported into the mitochondria, some researchers have designed a new synthetic OA analog, "minerval" with a modification that blocks the biological activity of fatty acids. Thus, enzymes involved in those processes may not recognize this modified fatty acid, so that its utilization as a source of energy would be decreased, and subsequently, its availability to modify cell signaling increased. Supporting the above-mention suggestion, they showed a markedly increase of the antiproliferative activity of minerval with respect to OA in human adenocarcinoma cells, with no apparent toxic effects. They additionally showed that exposure to minerval inhibits the growth of cancers in both animal models and cultured cells.30
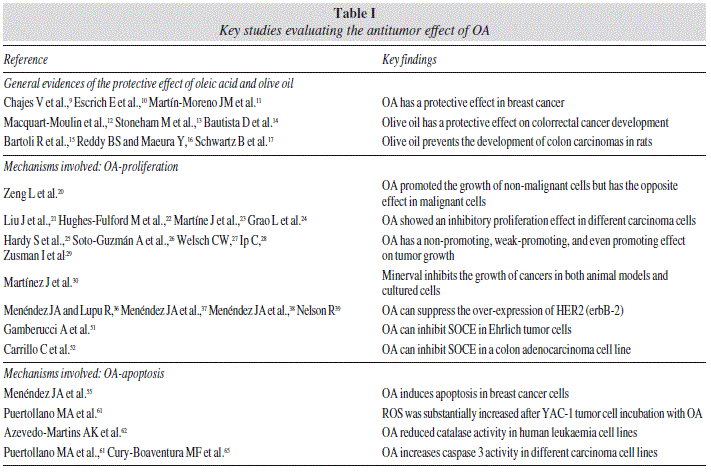
Several mechanisms have been proposed for the antiproliferative effect of unsaturated fatty acids. Among them, a reduction in the synthesis of eicosanoids derived from arachidonic acid could be considered to be involved in the growth inhibitory effect.31-33 Specific changes in gene expression patterns have been also suggested.34,35 In this sense, OA can suppress the over-expression of HER2 (erbB-2), a well-characterized oncogene which plays a key role in the etiology, invasive progression and metastasis in several human cancers.36-39 Finally, different studies have reported that unsaturated fatty acids can modulate the activity of the components of intracellular signaling.40-42 It is well established that Ca2+ is among the major intracellular factors involved in the signaling transduction pathways evoking cell growth and proliferation, and other key processes such as gene expression.43,44 Within Ca2+ mechanisms contributing to the increases in intracellular Ca2+ concentration ([Ca2+]i), particular attention has been paid to the role of Ca2+ entry through store-operated channels (SOC).45 Thus, store-operated Ca2+ entry (SOCE) has been involved in cell signaling occurring in non-excitable cells to evoke different cell processes including gene regulation and cell growth.46 Entry in cell cycle is preceded by SOC activation.47 In addition, the growth factor stimulation of cell proliferation is accompanied by increased activity and/or expression of TRP channels that are related to SOCE.48 Moreover, SOCE inhibition by different means abolishes tumor cell proliferation.49,50 It has been recently observed that the addition of PUFA to cells cultured in vitro, modifies the extent of SOCE.51 Thus, OA can inhibit SOCE in Ehrlich tumor cells, possibly because they intercalate into the plasma membrane and directly affect the activity of the channels involved.51 In addition, it has been recently reported a SOCE-inhibitory effect induced by oleic acid in a colon adenocarcinoma cell line.52
Oleic acid-apoptosis
Unsaturated fatty acids have been widely reported to induce apoptosis in several cell lines.53-55 The mechanisms behind cell death are numerous and involve a complex set of pathways.
In the apoptotic pathway, the collapse of the mitochondrial membrane potential is a common event that leads to mitochondrial dysfunction and the production of reactive oxygen species (ROS). In fact, increased ROS production has been associated with the induction of apoptotic cell death in different cells.56 According to these findings, exposure of neonatal cardiomyocytes to hydrogen peroxide or superoxide anion (O2·-) induces apoptosis.57 Whether apoptosis induced by fatty acids is associated with an increased production of ROS remains controversial and seems to depend on the type of fatty acid. Some researchers studied the role of ROS in palmitate-induced apoptosis in the neonatal rat cardiomyocyte and reported no evidence of ROS involvement.58 By contrast, docosahexaenoic acid (DHA), a n-3 PUFA, is able to disrupt the mitochondrial membrane permeability and induce ROS production in tumor cells.59,60 Regarding the effect of the MUFA, some researchers have reported that the production of ROS was substantially increased after YAC-1 tumor cell incubation with OA and other unsaturated fatty acids.61
Moreover, fatty acid may disturb the redox state of the cells not only owing to an increase in ROS generation as previously shown, but also due to a reduction in antioxidant enzyme activities. Thus, some researchers demonstrated that OA reduced catalase activity in human leukaemia cell lines.62
Apart from (and in line with) this increase in intracellular ROS production, unsaturated fatty acids-induced apoptosis has been reported to be mediated by an increase in caspase-3 activity in different carcinoma cell lines.59,61,63-65
In addition, alterations in intracellular Ca2+ homeostasis are commonly observed during apoptosis.66,67 It has been demonstrated that the depletion of the endoplasmic reticulum Ca2+ stores can directly induce apoptosis.68 Several reports have shown the ability of unsaturated fatty acids to induce Ca2+ release from the intracellular stores59,69,70 and to induce ROS generation with a subsequent cell death.59,71 This way, Aires et al. propose a model for the mechanism of action of DHA in which this fatty acid mobilizes Ca2+ from the intra-cellular pool and stimulates ROS production from the mitochondria, followed by downstream signaling, which will involve the activation of caspase-3, leading to chromatin fragmentation and apoptosis.59 However, evidence regarding the role of OA in these pathways is lacking.
In summary, research has demonstrated the advantageous effects of olive oil and OA on health at both the epidemiologic and cellular level. However, as far as we are concerned, little is known about the mechanisms by which OA could affect cell proliferation and cell death of cancer cells. Thus, much research needs to be conducted especially at the cellular level, to more fully understand the pathways by which OA could reduce cancer risk.
Acknowledgements
We thank Gonzalo Moreno for his support.
References
1. Kushi L, Giovannucci E. Dietary fat and cancer. Am J Med 2002; 113 (Suppl. 9B): 63S-70S. [ Links ]
2. Wiseman M. The second World Cancer Research Fund/American Institute for Cancer Research expert report. Food, nutrition, physical activity, and the prevention of cancer: a global perspective. Proc Nutr Soc 2008; 67 (3): 253-6. [ Links ]
3. Bartsch H, Nair J, Owen RW. Dietary polyunsaturated fatty acids and cancers of the breast and colorectum: emerging evidence for their role as risk modifiers. Carcinogenesis 1999; 20 (12): 2209-18. [ Links ]
4. Kolonel LN, Nomura AM, Cooney RV. Dietary fat and prostate cancer: current status. J Natl Cancer Inst 1999; 91(5): 414-28. [ Links ]
5. Rose DP. Effects of dietary fatty acids on breast and prostate cancers: evidence from in vitro experiments and animal studies. Am J Clin Nutr 1997; 66 (6 Suppl.): 1513S-22S. [ Links ]
6. Wynder EL, Cohen LA, Muscat JE, Winters B, Dwyer JT, Blackburn G. Breast cancer: weighing the evidence for a promoting role of dietary fat. J Natl Cancer Inst 1997; 89 (11):766-75. [ Links ]
7. Zock PL. Dietary fats and cancer. Curr Opin Lipidol 2001; 12(1): 5-10. [ Links ]
8. Trichopoulou A, Lagiou P, Kuper H, Trichopoulos D. Cancer and Mediterranean dietary traditions. Cancer Epidemiol Biomarkers Prev 2000; 9 (9): 869-73. [ Links ]
9. Chajes V, Thiebaut AC, Rotival M, Gauthier E, Maillard V, Boutron-Ruault MC, et al. Association between serum transmonounsaturated fatty acids and breast cancer risk in the E3N-EPIC Study. Am J Epidemiol 2008; 167 (11): 1312-20. [ Links ]
10. Escrich E, Solanas M, Moral R, Grau L, Costa I, Vela EE, R. Dietary lipids and breast cancer: Scientific clinical, anatomopathological and molecular evidences. Revista Española de Obesidad 2008; 6(3):129-38. [ Links ]
11. Martín-Moreno JM, Willett WC, Gorgojo L, Banegas JR, Rodríguez-Artalejo F, Fernández-Rodríguez JC et al. Dietary fat, olive oil intake and breast cancer risk. Int J Cancer 1994; 58(6): 774-80. [ Links ]
12. Macquart-Moulin G, Riboli E, Cornee J, Charnay B, Berthezene P, Day N. Case-control study on colorectal cancer and diet in Marseilles. Int J Cancer 1986; 38 (2): 183-91. [ Links ]
13. Stoneham M, Goldacre M, Seagroatt V, Gill L. Olive oil, diet and colorectal cancer: an ecological study and a hypothesis. J Epidemiol Community Health 2000; 54 (10): 756-60. [ Links ]
14. Bautista D, Obrador A, Moreno V, Cabeza E, Canet R, Benito E, et al. Ki-Ras mutation modifies the protective effect of dietary monounsaturated fat and calcium on sporadic colorectal cancer. Cancer Epidemiol Biomarkers Prev 1997; 6 (1): 57-61. [ Links ]
15. Bartoli R, Fernández-Banares F, Navarro E, Castella E, Mane J, Alvarez M, et al. Effect of olive oil on early and late events of colon carcinogenesis in rats: modulation of arachidonic acid metabolism and local prostaglandin E2 synthesis. Gut 2000; 46(2): 191-9. [ Links ]
16. Reddy BS, Maeura Y. Tumor promotion by dietary fat in azoxymethane-induced colon carcinogenesis in female F344 rats: influence of amount and source of dietary fat. J Natl Cancer Inst 1984; 72 (3): 745-50. [ Links ]
17. Schwartz B, Birk Y, Raz A, Madar Z. Nutritional-pharmacological combinations - a novel approach to reducing colon cancer incidence. Eur J Nutr 2004; 43 (4): 221-9. [ Links ]
18. Shaikh IA, Brown I, Wahle KW, Heys SD. Enhancing cytotoxic therapies for breast and prostate cancers with polyunsaturated fatty acids. Nutr Cancer 2010; 62 (3): 284-96. [ Links ]
19. Menéndez JA, del Mar Barbacid M, Montero S, Sevilla E, Escrich E, Solanas M, et al. Effects of gamma-linolenic acid and oleic acid on paclitaxel cytotoxicity in human breast cancer cells. Eur J Cancer 2001; 37 (3): 402-13. [ Links ]
20. Zeng L, Biernacka KM, Holly JM, Jarrett C, Morrison AA, Morgan A et al. Hyperglycaemia confers resistance to chemotherapy on breast cancer cells: the role of fatty acid synthase. Endocr Relat Cancer 2010; 17 (2): 539-51. [ Links ]
21. Liu J, Shimizu K, Kondo R. Anti-androgenic activity of fatty acids. Chem Biodivers 2009; 6 (4): 503-12. [ Links ]
22. Hughes-Fulford M, Chen Y, Tjandrawinata RR. Fatty acid regulates gene expression and growth of human prostate cancer PC-3 cells. Carcinogenesis 2001; 22 (5): 701-7. [ Links ]
23. Martínez J, Gutiérrez A, Casas J, Llado V, López-Bellan A, Besalduch J, et al. The repression of E2F-1 is critical for the activity of Minerval against cancer. J Pharmacol Exp Ther 2005; 315 (1): 466-74. [ Links ]
24. Girao LA, Ruck AC, Cantrill RC, Davidson BC. The effect of C18 fatty acids on cancer cells in culture. Anticancer Res 1986; 6 (2): 241-4. [ Links ]
25. Hardy S, St-Onge GG, Joly E, Langelier Y, Prentki M. Oleate promotes the proliferation of breast cancer cells via the G protein-coupled receptor GPR40. J Biol Chem 2005; 280 (14):13285-91. [ Links ]
26. Soto-Guzmán A, Navarro-Tito N, Castro-Sánchez L, Martínez-Orozco R, Salazar EP. Oleic acid promotes MMP-9 secretion and invasion in breast cancer cells. Clin Exp Metastasis 2010; 27 (7): 505-15. [ Links ]
27. Welsch CW. Relationship between dietary fat and experimental mammary tumorigenesis: a review and critique. Cancer Res 1992; 52 (7 Suppl.): 2040s-8s. [ Links ]
28. Ip C. Review of the effects of trans fatty acids, oleic acid, n-3 polyunsaturated fatty acids, and conjugated linoleic acid on mammary carcinogenesis in animals. Am J Clin Nutr 1997; 66 (6 Suppl.): 1523S-9S. [ Links ]
29. Zusman I, Gurevich P, Madar Z, Nyska A, Korol D, Timar B, et al. Tumor-promoting and tumor-protective effects of high-fat diets on chemically induced mammary cancer in rats. Anticancer Res 1997; 17 (1A): 349-56. [ Links ]
30. Martínez J, Vogler O, Casas J, Barcelo F, Alemany R, Prades J, et al. Membrane structure modulation, protein kinase C alpha activation, and anticancer activity of minerval. Mol Pharmacol 2005; 67 (2): 531-40. [ Links ]
31. Das UN. Essential fatty acids and their metabolites and cancer. Nutrition 1999; 15 (3): 239-40. [ Links ]
32. Karmali RA. Eicosanoids in neoplasia. Prev Med 1987; 16 (4):493-502. [ Links ]
33. Rose DP, Connolly JM, Rayburn J, Coleman M. Influence of diets containing eicosapentaenoic or docosahexaenoic acid on growth and metastasis of breast cancer cells in nude mice. J Natl Cancer Inst 1995; 87 (8): 587-92. [ Links ]
34. Sampath H, Ntambi JM. Polyunsaturated fatty acid regulation of genes of lipid metabolism. Annu Rev Nutr 2005; 25: 317-40. [ Links ]
35. Narayanan BA, Narayanan NK, Simi B, Reddy BS. Modulation of inducible nitric oxide synthase and related proinflammatory genes by the omega-3 fatty acid docosahexaenoic acid in human colon cancer cells. Cancer Res 2003; 63 (5): 972-9. [ Links ]
36. Menéndez JA, Lupu R. Mediterranean dietary traditions for the molecular treatment of human cancer: anti-oncogenic actions of the main olive oil's monounsaturated fatty acid oleic acid (18:1n-9). Curr Pharm Biotechnol 2006; 7 (6): 495-502. [ Links ]
37. Menéndez JA, Vázquez-Martín A, Colomer R, Brunet J, Carrasco-Pancorbo A, García-Villalba R, et al. Olive oil's bitter principle reverses acquired autoresistance to trastuzumab (Herceptin) in HER2-overexpressing breast cancer cells. BMC Cancer 2007; 7: 80. [ Links ]
38. Menéndez JA, Papadimitropoulou A, Vellon L, Lupu R. A genomic explanation connecting "Mediterranean diet", olive oil and cancer: oleic acid, the main monounsaturated fatty acid of olive oil, induces formation of inhibitory "PEA3 transcription factor-PEA3 DNA binding site" complexes at the Her-2/neu (erbB-2) oncogene promoter in breast, ovarian and stomach cancer cells. Eur J Cancer 2006; 42 (15): 2425-32. [ Links ]
39. Nelson R. Oleic acid suppresses overexpression of ERBB2 oncogene. Lancet Oncol 2005; 6 (2): 69. [ Links ]
40. Rao CV, Reddy BS. Modulating effect of amount and types of dietary fat on ornithine decarboxylase, tyrosine protein kinase and prostaglandins production during colon carcinogenesis in male F344 rats. Carcinogenesis 1993; 14 (7): 1327-33. [ Links ]
41. Marignani PA, Sebaldt RJ. Formation of second messenger diradylglycerol in murine peritoneal macrophages is altered after in vivo (n-3) polyunsaturated fatty acid supplementation. J Nutr 1995; 125 (12): 3030-40. [ Links ]
42. de Jonge HW, Dekkers DH, Lamers JM. Polyunsaturated fatty acids and signalling via phospholipase C-beta and A2 in myocardium. Mol Cell Biochem 1996; 157 (1-2): 199-210. [ Links ]
43. Berridge MJ. Calcium signalling and cell proliferation. Bioessays 1995; 17 (6): 491-500. [ Links ]
44. Kahl CR, Means AR. Regulation of cell cycle progression by calcium/calmodulin-dependent pathways. Endocr Rev 2003; 24 (6): 719-36. [ Links ]
45. Parekh AB, Putney JW, Jr. Store-operated calcium channels. Physiol Rev 2005; 85 (2): 757-810. [ Links ]
46. Parekh AB, Penner R. Store depletion and calcium influx. Physiol Rev 1997; 77 (4): 901-30. [ Links ]
47. Sugioka M, Yamashita M. Calcium signaling to nucleus via store-operated system during cell cycle in retinal neuroepithelium. Neurosci Res 2003; 45 (4): 447-58. [ Links ]
48. Golovina VA, Platoshyn O, Bailey CL, Wang J, Limsuwan A, Sweeney M et al. Upregulated TRP and enhanced capacitative Ca2+ entry in human pulmonary artery myocytes during proliferation. Am J Physiol Heart Circ Physiol 2001; 280 (2): H746-55. [ Links ]
49. Weiss H, Amberger A, Widschwendter M, Margreiter R, Ofner D, Dietl P. Inhibition of store-operated calcium entry contributes to the anti-proliferative effect of non-steroidal anti-inflammatory drugs in human colon cancer cells. Int J Cancer 2001; 92(6): 877-82. [ Links ]
50. Zitt C, Strauss B, Schwarz EC, Spaeth N, Rast G, Hatzelmann A et al. Potent inhibition of Ca2+ release-activated Ca2+ channels and T-lymphocyte activation by the pyrazole derivative BTP2. J Biol Chem 2004; 279 (13): 12427-37. [ Links ]
51. Gamberucci A, Fulceri R, Benedetti A. Inhibition of storedependent capacitative Ca2+ influx by unsaturated fatty acids. Cell Calcium 1997; 21 (5): 375-85. [ Links ]
52. Carrillo C, Cavia MM, Alonso-Torre SR. Oleic acid inhibits store-operated calcium entry in human colorectal adenocarcinoma cells. Eur J Nutr 2011: DOI: 10.1007/s00394-011-0246-8 [ Links ]
53. Habermann N, Schon A, Lund EK, Glei M. Fish fatty acids alter markers of apoptosis in colorectal adenoma and adenocarcinoma cell lines but fish consumption has no impact on apoptosis-induction ex vivo. Apoptosis 2010; 15 (5): 621-30. [ Links ]
54. Llor X, Pons E, Roca A, Alvarez M, Mane J, Fernández-Banares F, et al. The effects of fish oil, olive oil, oleic acid and linoleic acid on colorectal neoplastic processes. Clin Nutr 2003; 22 (1): 71-9. [ Links ]
55. Menendez JA, Vellon L, Colomer R, Lupu R. Oleic acid, the main monounsaturated fatty acid of olive oil, suppresses Her-2/neu (erbB-2) expression and synergistically enhances the growth inhibitory effects of trastuzumab (Herceptin) in breast cancer cells with Her-2/neu oncogene amplification. Ann Oncol 2005; 16 (3): 359-71. [ Links ]
56. Kamata H, Hirata H. Redox regulation of cellular signalling. Cell Signal 1999; 11 (1): 1-14. [ Links ]
57. von Harsdorf R, Li PF, Dietz R. Signaling pathways in reactive oxygen species-induced cardiomyocyte apoptosis. Circulation 1999; 99 (22): 2934-41. [ Links ]
58. Hickson-Bick DL, Sparagna GC, Buja LM, McMillin JB. Palmitate-induced apoptosis in neonatal cardiomyocytes is not dependent on the generation of ROS. Am J Physiol Heart Circ Physiol 2002; 282 (2): H656-64. [ Links ]
59. Aires V, Hichami A, Filomenko R, Ple A, Rebe C, Bettaieb A, et al. Docosahexaenoic acid induces increases in [Ca2+]i via inositol 1,4,5-triphosphate production and activates protein kinase C gamma and -delta via phosphatidylserine binding site: implication in apoptosis in U937 cells. Mol Pharmacol 2007; 72 (6): 1545-56. [ Links ]
60. Arita K, Kobuchi H, Utsumi T, Takehara Y, Akiyama J, Horton AA, et al. Mechanism of apoptosis in HL-60 cells induced by n-3 and n-6 polyunsaturated fatty acids. Biochem Pharmacol 2001; 62 (7): 821-8. [ Links ]
61. Puertollano MA, de Pablo MA, Alvarez de Cienfuegos G. Polyunsaturated fatty acids induce cell death in YAC-1 lymphoma by a caspase-3-independent mechanism. Anticancer Res 2003; 23 (5A): 3905-10. [ Links ]
62. Azevedo-Martins AK, Curi R. Fatty acids decrease catalase activity in human leukaemia cell lines. Cell Biochem Funct 2008; 26 (1): 87-94. [ Links ]
63. Diep QN, Touyz RM, Schiffrin EL. Docosahexaenoic acid, a peroxisome proliferator-activated receptor-alpha ligand, induces apoptosis in vascular smooth muscle cells by stimulation of p38 mitogen-activated protein kinase. Hypertension 2000; 36 (5): 851-5. [ Links ]
64. Kim HJ, Vosseler CA, Weber PC, Erl W. Docosahexaenoic acid induces apoptosis in proliferating human endothelial cells. J Cell Physiol 2005; 204 (3): 881-8. [ Links ]
65. Cury-Boaventura MF, Cristine Kanunfre C, Gorjao R, Martins de Lima T, Curi R. Mechanisms involved in Jurkat cell death induced by oleic and linoleic acids. Clin Nutr 2006; 25 (6): 1004-14. [ Links ]
66. McConkey DJ, Orrenius S. The role of calcium in the regulation of apoptosis. Biochem Biophys Res Commun 1997; 239 (2): 357-66. [ Links ]
67. Nicotera P, Orrenius S. The role of calcium in apoptosis. Cell Calcium 1998; 23 (2-3): 173-80. [ Links ]
68. Pan Z, Damron D, Nieminen AL, Bhat MB, Ma J. Depletion of intracellular Ca2+ by caffeine and ryanodine induces apoptosis of Chinese hamster ovary cells transfected with ryanodine receptor. J Biol Chem 2000; 275 (26): 19978-84. [ Links ]
69. Bonin A, Khan NA. Regulation of calcium signalling by docosahexaenoic acid in human T-cells. Implication of CRAC channels. J Lipid Res 2000; 41 (2): 277-84. [ Links ]
70. Gamberucci A, Fulceri R, Bygrave FL, Benedetti A. Unsaturated fatty acids mobilize intracellular calcium independent of IP3 generation and VIA insertion at the plasma membrane. Biochem Biophys Res Commun 1997; 241 (2): 312-6. [ Links ]
71. Puskas LG, Feher LZ, Vizler C, Ayaydin F, Raso E, Molnar E, et al. Polyunsaturated fatty acids synergize with lipid droplet binding thalidomide analogs to induce oxidative stress in cancer cells. Lipids Health Dis 2010; 9: 56. [ Links ]
![]() Correspondence:
Correspondence:
Sara R. Alonso-Torre.
Área de Nutrición y Bromatología.
Facultad de Ciencias.
Universidad de Burgos.
Pl. Misael Bañuelos s/n.
09001 Burgos. Spain.
E-mail: salonso@ubu.es
Recibido: 17-VI-2012.
Aceptado: 7-VIII-2012.














