INTRODUCTION
Obesity is defined as a chronic, complex, and multifactorial disease that is unfavorable to health and characterized by an excessive increase in body fat (1). Over the past decades, obesity in children has increased worldwide, with an eight-fold increase in children between 5-19 years of age within the period of 1975 and 2016. By 2016, the number of obese children and adolescents aged 5-19 years had increased to 50 million girls and 75 million boys (2). According to the 2018 Health and Nutrition Survey conducted in Mexico, there was a 35.6 % combined prevalence of overweight and obesity in the school population (18.1 % overweight and 17.5 % obesity). Said prevalence was higher in boys (37.8 %) than in girls (33.4 %) (3).
Obese individuals are known to be prone to vitamin D deficiency, because vitamin D is a liposoluble vitamin that is sequestered in adipose tissue. Vitamin D deficiency and obesity are two interrelated global epidemics that affect school-aged children. Hypovitaminosis D and vitamin D deficiency have a high prevalence worldwide, in all age groups. Recent data on 4691 Mexican children, 1-11 years of age, were analyzed. Vitamin D deficiency was found in 17.2 % of school-age children and was positively associated with the body mass index (BMI) (4). Another study performed by our group on Mexican children and adolescents reported 33.5 % insufficiency and 11.5 % deficiency (5). Clearly, obesity and hypovitaminosis D are health problems that have been described in pediatric populations worldwide.
Many studies have provided evidence of a strong association between vitamin D and cardiovascular risks, especially involving blood pressure. Vitamin D deficiency could be linked to the pathophysiology of hypertension, through its influence on the renin-angiotensin system (6). Despite the existing information about the mechanisms through which vitamin D protects against cardiovascular disease, particularly in the field of pediatric research, studies regarding the association of vitamin D and blood pressure are controversial (7), and a recent systematic review reported a lack of association between vitamin D status and blood pressure in children and adolescents (8).
On the other hand, childhood obesity is a risk factor for the development of hypertension, but other modifiable factors may also be involved in its development, such as physical activity and dietary factors, in which vitamin D could play an important role (9). In some studies, obese children with lower vitamin D levels have shown increased odds for hypertension, and that association may vary among age groups and sex (10-12). Therefore, the aim of the present study was to examine the relation between serum 25(OH)D and blood pressure in schoolchildren presenting with obesity.
MATERIAL AND METHODS
STUDY POPULATION
A cross-sectional study was conducted on children between 6 and 12 years of age, diagnosed with obesity, that attended four elementary schools, within the time frame of August 2017 and August 2018. The inclusion criteria were participation acceptance, statements of informed consent signed by the parents, and obesity determined by the WHO criteria (13). The exclusion criteria were children with diabetes, hypothyroidism, or any disease related to obesity, and children receiving treatment with antiepileptic drugs, corticoids and/or nutritional supplements, or whose anthropometric data were missing. The formula for calculating sample size was based on a correlation (14). The expected correlation between the two variables was -0.27 (15), with a statistical power of 80 % and an alpha of 95 %. The estimated sample size was 135 schoolchildren. Convenience sampling, as opposed to random sampling, was carried out. The present study was conducted in compliance with the Declaration of Helsinki and the Research Ethics Committee of the Hospital Regional Universitario in Colima, Mexico (Registration number: 2016/1/CR/CL/PD/113).
DATA COLLECTION PROCEDURE
Anthropometric data
All measurements were carried out by trained technicians and performed according to international anthropometric standards, following the official anthropometry manual of the International Society for the Advancement of Kinanthropometry (ISAK, 2001). The measurements were taken by accredited nutrition researchers, with ISAK level 1 international anthropometrism certifications. For weight and height measurements, 100 g precision and 200 kg capacity portable scales (SECA model 813®) and 1-mm precision and 2.05-m capacity portable stadiometers (SECA model 213®) were used.
The children were measured according to standard procedures, wearing lightweight clothing and no shoes. BMI was calculated as weight (kg) divided by height squared (m2). The definitions of obesity were based on the age-specific BMI Z scores established by the 2007 WHO criteria (13).
Blood pressure (BP)
BP was consecutively measured 3 times, using an integrated aneroid sphygmomanometer kit with a stethoscope (RM 1807001E®). An appropriately sized cuff was selected, according to arm circumference, to ensure that the length and width of the bladder inside the cuff encircled 80 % and 40 % of the upper arm, respectively. The stethoscope was placed over the brachial artery pulse, proximal and medial to the cubital fossa, below the bottom edge of the cuff. It was taken with the child in a sitting position, using the left arm. Three readings were taken, at 10-minute intervals, obtaining the average. Systolic blood pressure (SBP) was identified using phase I of the Korotkoff sound, and diastolic blood pressure (DBP) was identified by phase V of the Korotkoff sound. SBP and DBP classifications were made, according to blood pressure reference tables specific for age, sex, and height. Normal values were considered < 90th percentile, elevated blood pressure ≥ 90th to < 95th percentiles, and hypertension ≥ 95th percentile (16,17).
Quantification of serum levels of 25[OH]D
Prior to blood sample collection, the participants fasted 8 to 12 hours. The blood was drawn by a biochemistry specialist, following the standards of the Clinical and Laboratory Standards Institute (CLSI). Each blood sample was centrifuged at 3000 rpm for 10 min to obtain the serum, which was frozen at -70 °C on site and shipped on dry ice to the multidisciplinary laboratory of the medical school, until analysis. Vitamin D levels were measured by enzyme immunoassay, using the ELISA test (Inmunodiagnostik AG, Bensheim, Germany, Cat. 2154). The concentrations were classified according to the cutoff point established by the Clinical Practice Guidelines of the Endocrine Society as follows: < 20 ng/mL of 25-OH-D corresponded to deficient levels; 21 to 29 ng/mL to insufficient vitamin D; ≥ 30 to < 100 ng/mL to sufficient vitamin D; and ≥ 100 n/mL to possible vitamin D poisoning or excess vitamin D (18).
STATISTICAL ANALYSIS
To determine the optimal statistical test to be used, the normality of data was confirmed or ruled out, using the Kolmogorov-Smirnov test, and the equality of variances was confirmed, using the Levene's test. Descriptive analyses were performed. The continuous data were presented as means ± standard deviations and the categorical variables were presented as numbers and percentages. A one-way analysis of variance was utilized to compare the continuous data between groups. For multiple comparisons, the Tukey's honest significant difference test was used. The chi-square test was utilized to compare the categorical variables between groups. Finally, the Pearson correlation coefficient was used to determine the relationship between 25(OH)D and blood pressure. We conducted a multiple regression analysis to identify the interaction of the independent variables (age, BMI and 25(OH)D levels) with the SBP and DBP. The SPSS v25.0 software was utilized for the statistical analysis and statistical significance was set at a p < 0.05.
RESULTS
A total of 256 obese children, 6-12 years of age (123 [48.0 %] females and 133 [51.9 %] males), that met the selection criteria were included. The mean age was 8.7 ± 2.7 years, and the mean 25(OH)D level was 15.8 ± 8.8 ng/mL. The prevalence rates of 25(OH)D deficiency, insufficiency, and sufficiency were 60 [23.4 %], 134 [52.3 %], and 62 [24.2%], respectively. Additionally, 101 [39.4 %] of the children analyzed had normal blood pressure, 28 [10.9 %] had elevated blood pressure, and 127 [49.6 %] presented with hypertension. Table I summarizes the qualitative variable distribution in the entire study population, according to the levels of 25(OH)D. The prevalence of elevated blood pressure and hypertension, regarding SBP, was found to be significantly higher in the deficient and insufficient group vs the sufficient group.
Table I. Distribution of qualitative variables according to the levels of 25(OH)D in schoolchildren with obesity
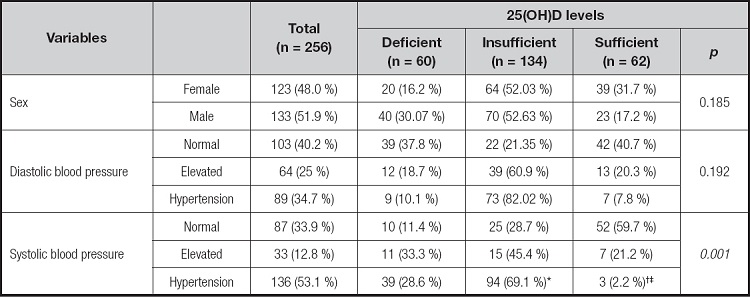
Data are shown as n (%). The chi-square test was used.
*p ≤ 0.05 when deficient vs insufficient,.
†deficient vs sufficient, and.
‡insufficient vs sufficient were compared. Deficient: 25(OH)D level < 20 ng/mL; insufficient: 25(OH)D level = 21 to 29 ng/mL; sufficient: 25(OH)D level ≥ 30 ng/mL (23).
Table II summarizes the mean values and quantitative variables, according to the serum levels of 25(OH)D. The SBP values in the deficient and insufficient groups were higher than those of the sufficient group, with statistical significance.
Table II. Distribution of quantitative variables, according to the levels of 25(OH)D in schoolchildren with obesity
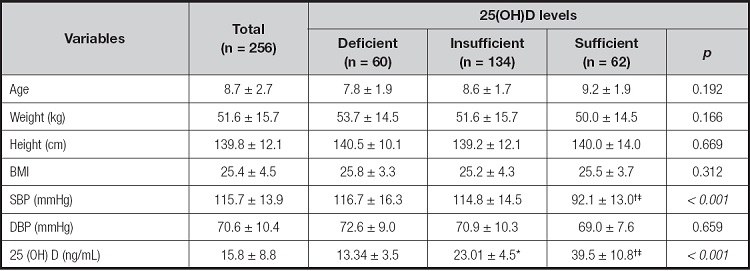
Data are shown as mean ± standard deviation (ANOVA, Tukey's post-hoc). BMI: body mass index; SBP: systolic blood pressure; BDP: diastolic blood pressure.
*p ≤ 0.05 when deficient vs insufficient;.
†deficient vs sufficient, and.
‡insufficient vs sufficient were compared. Deficient: 25(OH)D level < 20 ng/mL; insufficient: 25(OH)D level = 21 to 29 ng/mL; sufficient: 25(OH)D level ≥ 30 ng/mL (23 ).
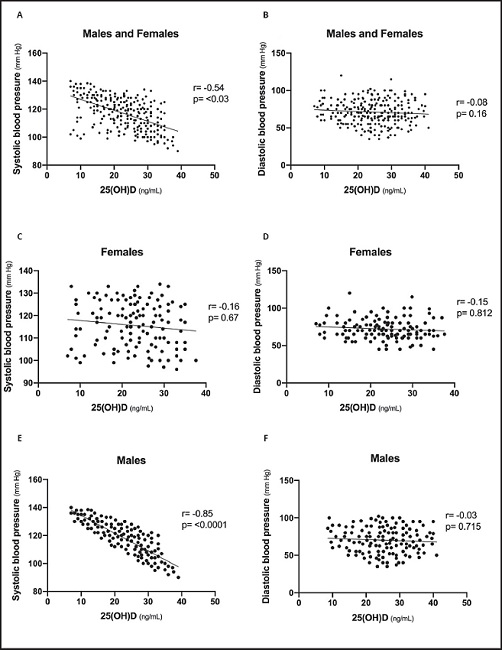
Regarding the correlations between 25(OH)D levels and blood pressure, a moderate negative correlation was found between 25(OH)D levels and SBP (r = -0.54 and p = 0.03), and no correlation was found with DBP (r = -0.08 and p = 0.16). When analyzed by sex, a strong and significant inverse correlation between 25(OH)D levels and SBP was exclusively observed in males (r = -0.85 and p ≤ 0.001); no correlation was found in relation to female sex (systolic, r = -0.16 and p = 0.67; diastolic, r = -0.15 and p = 0.812). Figure 1 presents scatter plots of 25(OH)D, with values of SBP and DBP, respectively.
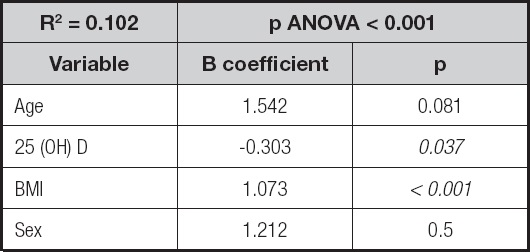
Finally, a multiple regression analysis was conducted, predicting the SBP values. The overall model accounted for approximately 10.2 % of the variance in SBP values (F (7, 357) = 18.322, p = 0.001), with BMI and 25(OH)D levels as significant predictors (Table III).
DISCUSSION
The present study examined the relationship between serum 25(OH)D levels and BP in schoolchildren with obesity. The results showed a significant correlation between SBP and 25(OH)D levels in the school-age boys. When analyzing the variables in multiple regression, the significant predictor variables were 25 (OH) D levels and BMI. There was no correlation in girls or with DBP. Our results are consistent with previous studies that identified an association between 25(OH)D deficiency and hypertension. For example, in a representative sample of 6275 children and adolescents (1-21 years of age) from the National Health and Nutrition Examination Survey, Kumar et al. found an association between 25(OH)D insufficiency/deficiency and high systolic blood pressure, with a 2.24-fold greater risk of developing high systolic blood pressure. Kao et al. found a 4-fold greater increase in the OR of having high BP for children in the lowest 25(OH)D quintile, compared with those in the highest quintile, and it remained significant after BMI adjustment (10). Recent research performed on 6091 Chinese children and adolescents identified a significant negative correlation between SBP and 25(OH)D levels. When analyzed by sex, it remained significant only in girls, contrary to what we found. Nutritional status was not analyzed (19). In the same study, those authors found a clearly higher prevalence of hypertension in girls with inadequacy and deficiency of 25(OH)D status than in girls with adequate status, which was not observed in boys. In comparison with our results, we found a higher prevalence of elevated blood pressure and hypertension in the groups with insufficiency and deficiency of 25(OH)D, and when solely analyzing by sex, there was a significant correlation in boys. More research must be carried out on the pediatric population, to determine whether sex affects 25(OH)D status, and how that status influences blood pressure.
The link between low serum 25(OH)D concentrations and cardiovascular risk, in children and adolescents, has been described in observational studies, identifying an association with endothelial dysfunction, increased arterial stiffening, and an increase in intima-media thickness. A negative correlation between 25(OH)D levels and SBP and DBP values has also been found, as well as suppression of the renin-angiotensin-aldosterone system and parathyroid hormone (PTH), which diminishes sympathetic activity and stabilizes calcium transport in the vascular smooth muscle. An inverse association of 25(OH)D with systemic markers of oxidative stress has also been described (6,10,11,20,21).
Interventional studies supplying vitamin D have had inconsistent results, mainly because of the doses given, supplementation duration, and the cutoff points used for vitamin D deficiency. For example, a randomized clinical trial conducted by Dong et al. randomly assigned adolescents to the control group (400 IU/day of vitamin D3) or the experimental group (2000 IU/day of vitamin D3) for 16 weeks. They found that optimal vitamin D status was achieved after 16 weeks of 2000 IU/day of vitamin D3, favorably improving aortic stiffness in black youths (20).
Regarding nutritional status, we found that vitamin D insufficiency or deficiency was clearly a frequent issue in the obese population. Such findings have already been described in observational and interventional studies performed on adult and pediatric populations, in which an inverse association between adiposity (total and central) and vitamin D levels has been found. A study conducted on prepubertal Chilean children found that those with suboptimal serum 25(OH)D had a higher risk (2 or 3-fold) for obesity (22). Another study carried out on Spanish children reported higher body weight, BMI, waist measurement, and waist-to-height ratio in the group of children with vitamin D insufficiency, compared with the group with adequate levels of vitamin D (23). The mechanisms related to vitamin D and obesity have been described as follows: vitamin D deficiency increases PTH levels, leading to an intracellular calcium flux in adipocytes, enhancing lipogenesis and promoting weight gain (21). However, obesity may also be the consequence of poor vitamin D status, since vitamin D is sequestered in a larger body pool of adipose tissue in obese individuals, leading to less bioavailable 25(OH)D in the circulation (24). Other associated factors are low sun exposure and sedentary activities in obese children (25).
Importantly, the prevalence of elevated systolic-diastolic blood pressure and hypertension in our study population was present in half of the children with obesity. A study performed on children and adolescents (2-18 years of age) attending a pediatric weight management clinic identified elevated blood pressure and stage I hypertension in 14 % and 25 % of the cases, respectively (26), whereas a study conducted on obese Spanish schoolchildren, 4-6 years of age, reported a prevalence of elevated blood pressure and hypertension of 12.3 % and 18.2 %, respectively (27). In their study, Yang et al. found that the status of overweight and obesity doubled the risk of high blood pressure (28). In another study performed on adolescents, those authors reported that obesity rates were highest among African American and Hispanic adolescents, and that the Hispanic youths had the highest rates of hypertension (12). A study conducted on Chinese children reported that boys presenting with adiposity had a greater risk of high blood pressure than girls (28). A recent study performed on Hungarian children (8624) identified a greater frequency of high BP in children with obesity, with an OR of 3.6 (29). Obesity is a well-known modifiable risk factor that has been associated with hypertension in children and adolescents, and factors such as race could also be important.
Among the limitations of our study is the fact that we did not measure other factors that could contribute to 25(OH)D levels, in relation to cardiometabolic disorders, such as sun exposure, pubertal stage, skin phototype, vitamin D intake, physical activity, and seasonal variations. However, Colima has a predominantly sub-humid warm climate (86%) with an average annual temperature of 25 °C (max 30°, min 18°), as described by the National Institute of Statistics and Geography (30), so we believe that the time the serum was taken did not affect the serum 25(OH)D levels.
CONCLUSION
To the best of our knowledge, this is the first Mexican study to evaluate the association between BP and serum 25(OH)D levels in school-age children with obesity, in which 25(OH)D levels had an inverse correlation with SBP. Therefore, clinical trials should be conducted to demonstrate whether vitamin D supplementation could be a therapeutic option in children that present with BP values ≥ 90 percentile and vitamin D insufficiency or deficiency.