Introduction
The liver is one of the most important organs for the metabolic activity that the organism develops, synthesizes fatty acids, stores glycogen, forms lipoproteins, synthesizes plasma proteins, forms urea, synthesizes coagulation factors, catabolizes and excretes hormones, detoxifies endogenous and exogenous substances, forms bile, maintains the hydroelectrolytic balance, etc.1. Hepatocytes constitute 80% of the liver’s cell population, giving the liver great regenerative capacity, but when it is constantly attacked as in alcohol intake, viral infections, it progresses to irreversible liver damage2.
Alcohol is a direct liver toxin, intervening facilitating factors and comorbidities such as gender, inherited factors and immunity3. In Europe, liver diseases are a serious health problem, mainly cirrhosis and liver cancer4, in the United States, deaths due to liver disease and liver cancer have increased5, in Peru, liver diseases were one of the first ten causes of mortality in 20176.
In recent years, plants such as Silybum marianum “milk thistle”, Fumaria officinalis L. “fumaria”, Peumus boldus M. “boldo”, among other plants, are used to treat liver diseases7. Among these herbal formulations, silymarin obtained from Silybum marianum is highly effective for liver disorders, increasing the survival of patients with alcohol-induced cirrhosis8.
Niphidium albopunctatissimum Lellinger (Polypodiaceae) “calaguala” is a fern found in the Andes Mountains, from Colombia to southern Bolivia, at altitudes between 500 and 2720 m9. Although, popular uses of N. albopunctatissimum report that rhizomes are used for its anti-inflammatory, astringent, depurative, hepatic and emmenagogue properties10, studies evidencing the mentioned properties are not found in the scientific literature.
Liver diseases are reaching increased prevalence rates over the years and alcohol consumption is one of the most important risk factors for these pathologies. In addition, many pharmacological treatments have side effects related to liver problems, so the use of alternative medicine with N. albopunctatissimum Lellinger could contribute to the prevention or treatment of liver disease. The objective of this study was to identify phytoconstituents and evaluate the effect of Niphidium albopunctatissimum Lellinger on alcoholic hepatoxicity induced in Rattus norvegicus var. albinus.
Materials
Plant material
4 kg of Niphidium albopunctatissimum Lellinger rhizomes, collected in Queropuspo sector, Cospán district, Cajamarca Province, Peru, at an altitude of 3000 m was used. A sample was identified at Truxillense Herbarium (HUT) of the National University of Trujillo (UNT) and deposited with the code 58842.
Animal material
Thirty male Rattus norvegicus var. albinus 3-month-old, between 170 and 200 g of body weight, acquired at the Bioterium of the Faculty of Pharmacy and Biochemistry of the UNT were used. They were maintained at a temperature of 18 ± 22 ° C, with light / dark cycles of 12/12 h, fed with standard diet and water ad libitum.
Methods
Sample and fluid extract preparation
The rhizomes of Niphidium albopunctatissimum Lellinger were mechanically ground until obtaining small particles which were sieved through sieve No. 0.10 (2 mm diameter), stored tightly in amber and wide-mouth glass jars after. 100 g of drug were moistened with 70 ° GL water and ethanol for half an hour, placed in the percolator, macerated by adding water for 48 hours. 75 mL of fluid extract was collected, which was stored in amber bottle. The second successive portion of 25 mL was transferred to another bottle, extracted until the drug was used up and 230 mL was collected, which was mixed with the volume of the second extraction and concentrated to 25 mL11. The final amount of fluid extract was 100 mL.
Phytochemical screening
A volume of the fluid extract was separated to evaporate the solvent, the residue was partitioned equally into two porcelain capsules and dissolved with 5 mL of ethanol and water to be subsequently treated according to the phytochemical march of the Drop Test. Chemical identification, coloring and/or precipitation reactions were performed to determine the presence of secondary metabolites: steroids and triterpenes (Liebermann-Burchard’s test), flavonoids (Shinoda’s test), phenolic compounds (Ferric Chloride Test), saponins (Foam test), tannins (Gelatin test), alkaloids (Dragendorff, Hager, Mayer and Wagner's tests), anthocyanidins (Rosenheim's test), anthraquinones and naphthoquinones (Borntrager’s test)11.
Experimental design
Induction of alcoholic hepatoxicity and study groups
12 hour fasted rats were randomly selected to conform 5 groups of 6 animals each. Four were administered 56% ethanol (w/v) at doses of 7.6 mL/kg bw every 12 hours for 7 days12, except normal group.
Normal group (healthy rats): 7.5 mL/kg bw of boiled water every 12 h for fourteen days. Control group: with hepatoxicity and 7.5 mL/kg bw of boiled water every 12 hours for another seven days, completing fourteen days. Curative group: hepatotoxicity and fluid extract of N. albopunctatissimum Lellinger (500 mg/kg bw) every 12 hours for another seven days. Standard group: hepatotoxicity and silymarin (100 mg/kg bw)13 every 12 hours for seven days. Preventive group: fluid extract of N. albopunctatissimum L. (500 mg/kg bw) for 7 days, and after from day 8 to 14, first the fluid extract and thirty minutes later the alcohol for induction. In all cases, the administration was orally by gavage.
Transaminases determination
In all groups, baseline levels of fasting GPT transaminase were measured, then at 7 and 14 days of the study, blood was drawn from the tail vein of albino rat by puncture. The enzymatic method of Wiener Lab for transaminases was used14.
Histopathological study
At the end of the 14 days, the animals were sacrificed and the livers were removed for the respective studies. A portion of the liver of each albino rat was fixed in 10% formalin, infiltrated in paraffin, 6-micron thick blocks were cut and stained with hematoxylin and eosin for subsequent microscopic reading.
Ethical aspects
All procedures were performed in accordance with the protocols approved by the Ethics Committee for animal research of the UNT, (Res. Cons. Univ. No. 0247-2016 / UNT) and the Guide for the care and use of animal laboratory15.
Statistical analysis
Averages and standard deviation for serum GPT transaminase levels were calculated. The comparative study was performed using two-way analysis of variance (ANOVA) followed by Tukey HSD Test using the statistical package SSPS v.22. P values <0.05 were considered statistically significant.
Results and Discussion
Phytochemical screening of the rhizomes from N. albopunctatissimum Lellinger reports the presence of leucoanthocyanidins, flavonoids, tannins, polyphenols, saponins (Table 1), showing a greater presence of polyphenols.
Table 1. Phytoconstituents of the rhizomes from Niphidium albopunctatissimum L.
| Tests | Secondary metabolites | Extract Eta | Extract Ab |
|---|---|---|---|
| Dragendorff | Alkaloids | - | - |
| Mayer | Alkaloids | - | - |
| Wagner | Alkaloids | - | - |
| Hager | Alkaloids | - | - |
| Börntrager | Quinones | - | - |
| Liebermann-Bouchard | Triterpenes/ steroids | - | - |
| Rosenheim | Leucoanthocyanidins | - | ++ |
| Shinoda | Flavonoids | - | ++ |
| Foam | Saponins | + | - |
| Ferric Chloride | Polyphenols | +++ | +++ |
| Gelatin | Tannins | - | ++ |
aEt: ethanolic, bA: aqueous. Presence: (+), Absence: (-). Intensity: low (+), moderate (++), high (+++)
Figure 1 shows that on day 14 of the study, the serum GPT transaminase values were significantly decreased in the preventive group and the standard group, compared to the control group (p <0.01). It is also evident that the curative group has lower serum values than the control group (p <0.05). The GPT enzyme is a transaminase and is located primarily at the cytological level in the hepatocyte, giving it greater specificity, and when the liver suffers an injury, GPT is released from the liver cells, increasing its serum levels. It serves as a fairly specific indicator of liver status16. The decrease in serum GPT values found in our study is consistent with those reported in other investigations with hepatoprotective medicinal plants17,18, especially the fact that pretreatment with the extract may be effective in reducing serum GPT before drinking alcohol17.

Figure 1. Averages of GPT (IU/L) transaminase concentrations at baseline, 7 days post hepatoxicity induction and 14 days post treatment in the study groups. The values in each group (n = 6) are expressed as the mean ± D.S.M. (Two-way ANOVA / Post Hoc HSD from Tukey); * p <0.05; ** p <0.01: expresses statistically significant difference.
The hepatotoxic action of ethanol in control group is evident, since the absorbed ethanol performs its biotransformation process mainly in the liver, at a rate of 10 mL hour19; its metabolism is mainly carried out by the enzymes alcohol dehydrogenase (ADH), aldehyde dehydrogenase (ALDH) and cytochrome P450 (CYP2E1)20. The first phase is performed by gastric ADH, then in the liver, ADH metabolizes 80% ethanol to acetaldehyde, which is a highly toxic metabolite. Simultaneously there is reduction of the nicotine-adenine-dinucleotide (NAD) cofactor to reduced nicotine-adenine-dinucleotide (NADH); Acetaldehyde is converted to acetate at the mitochondrial level by ALDH (3). The contrast between a healthy liver (Fig.2A) and another with alcoholic hepatoxicity (Fig.2B) is observed, with hepatocytes with cloudy cytoplasm and fat drops, several in degeneration and chromatin dissolution. ADH causes excess NADH, altering the redox balance, which favors the action of xanthine oxidase, which during the degradation of purines releases oxygen free radicals, the basis of ethanol-induced damage3.
On the other hand, the hepatoprotective action of silymarin is shown in Fig. 1 and Fig. 3A, hepatocytes with normal contours, nucleus and cytoplasm are observed. Silymarin is a complex mixture of flavonolignans, which stabilizes cell membranes, stimulates detoxification pathways, stimulates of liver tissue regeneration21, increases cell vitality, reduces lipid peroxidation and cell necrosis22.
It is also observed (Fig. 3 B and C) that when N. albopunctatissimum is administered prior to alcohol induction, liver protection is greater than when administered as a curative treatment. The flavonoids, tannins, polyphenols and anthocyanidins that this plant contains could explain its hepatoprotective effect, since flavonoids inhibit enzymes that generate reactive oxygen species (ROS) such as microsomal monooxygenase, glutathione-S transferase, NADH transferase, etc.23; polyphenols decrease ROS levels by trapping and scavenging free electrons forming a phenoxy radical, less harmful to cells24) and cyanidin 3- glycoside has been shown to be able to decrease the level of accumulated free radicals in the brain by effect of ethanol and decrease lipid peroxidation25 protecting against alcoholic toxicity.

Figure 2. Rat liver. A) Normal group, healthy rats: radially to the central vein (cv) the hepatocyte (inset) and sinusoid plaques (arrows) flow. Hepatocyte nuclei and cytoplasm (circle) with normal staining and appearance. B) Control Group: hepatoxicity. Some hepatocytes with well-defined nucleus and nucleolus, others with cloudy cytoplasm and with fat drops (arrows), several in degeneration and dissolution of chromatin (*), necrosis. H&E.400x.
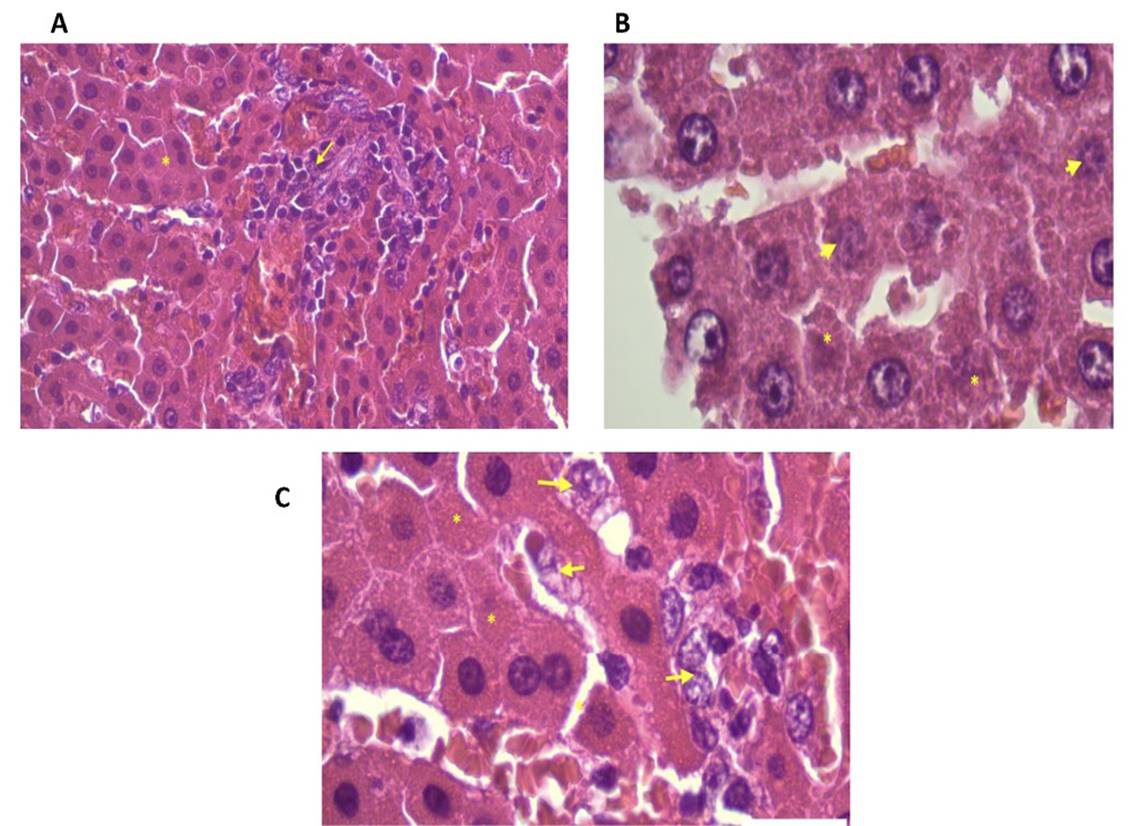
Figure 3. Rat liver. Treatments. A) Standard Group (silymarin). Hepatocytes with contours, nucleus and cytoplasm of normal appearance (*). Focus of inflammatory reaction (arrow). H&E 400x, B) Curative Group (N. albopunctatissimum). Some hepatocytes in degenerative process (arrow), many show normal-looking cytoplasm and others denote cell death (*). H&E 1000x, C) Preventive group (N. albopunctatissimum). Hepatocytes with nucleus and nucleolus with intense tintorial affinity, evidence of normality, few in degenerative process (arrow) and very few in degeneration (*). H&E 1000x.
Conclusion
The fluid extract of Niphidium albopunctatissimum Lellinger may have hepatoprotective potential by significantly lowering the plasma levels of GPT transaminase and preserving the hepatic architecture of albino rats against induced alcoholic toxicity. Further studies are planned to elucidate the specific chemical structures of polyphenols, tannins and flavonoids to identify the phytochemicals responsible for the hepatoprotective effect.














