Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Archivos Españoles de Urología (Ed. impresa)
versión impresa ISSN 0004-0614
Arch. Esp. Urol. vol.62 no.3 abr. 2009
Implantation technique of the artificial urinary sphincter "FlowSecureTM" in the bulbar urethra
Técnica de implantación del esfínter urinario artificial "FlowSecureTM" en uretra bulbar
Juan E. Bestard Vallejo, Fernando García Montes1, Lluis Cecchini Rosell, Carme Mir Maresma and Juan Morote Robles.
Department of Urology. Hospital Universitari Vall d'Hebron. UAB. Barcelona. Spain.
1Department of Urology. Hospital Universitari Son Dureta. UIB. Palma de Mallorca. Spain.
SUMMARY
Objectives: Artificial urinary sphincter "FlowSecureTM" is a prosthesis designed for stress urinary incontinence that has achieved excellent results. Although implantation is easy, some urologist used to other prosthesis may find difficult the change to this new technique. This article shows how easily and quickly this new sphincter can be implanted and discuss the differences with the artificial sphincter AMS-800TM.
Methods: Following the case of a patient who was implanted artificial urinary sphincter "FlowSecureTM" in our center we describe with pictures the technique of implantation and give some advices to make this quicker and easier.
Results: Surgical time was 90 minutes. Patient presented uneventful recovery, urethral catheter was removed 24 hours after surgery and discharge was done 72 hours after surgery. Three months after implantation patient describes complete resolution of his stress urinary incontinence.
Conclusions: Artificial urinary sphincter "FlowSecureTM" is easy and quick to implant, and as its use is extended we would compare if results are better than those of the AMS-800TM model.
Key words: Artificial urinary sphincter. Urinary Stress Incontinence. Surgical Technique.
RESUMEN
Objetivo: El esfínter urinario FlowSecureTM es una prótesis para la incontinencia urinaria de esfuerzo que ha proporcionado unos excelentes resultados hasta el día de hoy. Si bien su colocación es sencilla, los urólogos acostumbrados a la colocación de otros tipos de prótesis pueden encontrar inconveniente el cambio a esta nueva técnica. Este artículo pretende demostrar que este nuevo esfínter se puede colocar de forma rápida y sencilla, así como discutir las diferencias respecto del modelo AMS-800TM.
Métodos: A raíz de un paciente al que se colocó el esfínter urinario FlowSecureTM en nuestro centro se describe mediante dibujos la técnica de colocación y se apuntan ciertos consejos prácticos que hacen más fácil y rápida su colocación.
Resultado: El tiempo quirúrgico fue de 90 minutos. El paciente presentó un postoperatorio correcto, retirándose la sonda vesical a las 24 horas y siendo dado de alta a las 72 horas. A los 3 meses de la intervención el paciente refiere resolución completa de su incontinencia urinaria de esfuerzo.
Conclusiones: El esfínter urinario FlowSecureTM resulta una prótesis de colocación rápida y sencilla, y a medida que su utilización se vaya extendiendo podremos valorar si sus resultados a largo plazo son mejores que los del modelo AMS-800TM.
Palabras clave: Esfínter urinario artificial. Incontinencia urinaria de esfuerzo. Técnica quirúrgica.
Introduction
Artificial urinary sphincter "FlowSecureTM" is a prothesis for stress urinary incontinence designed by M.D. Cragg and A.R. Mundy in the Institute of Urology and Nephrology in London. Their aim was to improve deficiencies observed in previous models without compromising comfort and continence for the patient (1). Its implantation is easy and quick, but urologist used to the only model disposable for the last 23 years (American Medical System's AMS-800TM ) may find difficult learning the implantation technique of "FlowSecureTM".
We have had the chance of using FlowSecureTM in a 71 years old patient presenting urinary incontinence to minimal stress 2 years after laparoscopic radical prostatectomy performed in our center. Here we show using pictures and in an easy way which are the steps to implant this new urinary sphincter.
Material and methods
Parts of the Prothesis
Urinary prothesis FlowSecureTM consists on 4 parts which are: an adjustable pressure-regulating balloon, a stress relief reservoir, a control pump and valve assembly unit with self-sealing port and a urethral cuff. It's recommended to pump the prothesis before its implantation because it may block due to the sterilization process.
The valve assembly unit allows free flow of liquid in direction to the stuff but prevents its return. When patient press the pump passage of the liquid is momentary altered, lowering pressure in the cuff. Originary direction of flow is recovered when compression on the pump stops.
FlowSecureTM is accompanied by a plastic trocar and its obturator, which allows transposai of urethral cuff between Retzius space and perineum, and a tube of glue for temporary fixation of the belt over the cuff when adjusting it (2).
Additional elements
Additional material needed for prothesis implantation are: 16 Ch silicon urethral catheter, bookwalter retractor, 2 rubber-shod mosquito forceps (to occlude connection tubes), 2 sterile syringes, one (a 10 mL syringe) for decompressing the sphincter and the other (a 2 mL syringe) to apply the glue, a 25G insulin needle with a length of 15 mm (to extract liquid from the prothesis through its self-sealing port), a Penrose drainage and a 4-0 prolene suture(3).
Prothesis implantation
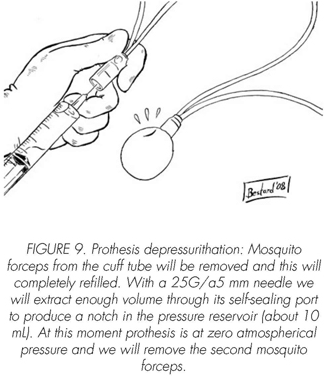
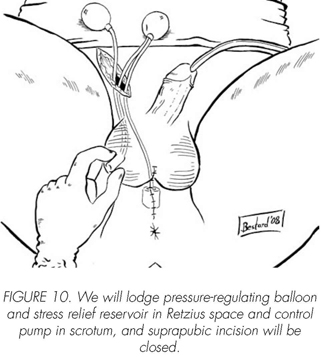
See pictures 1 to 10.
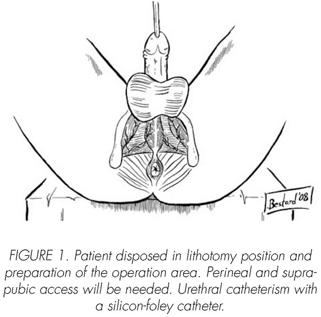
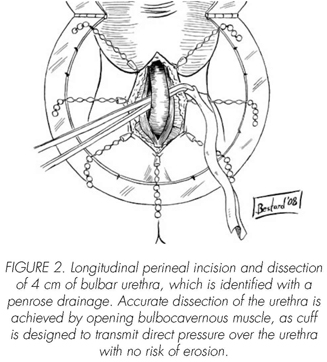
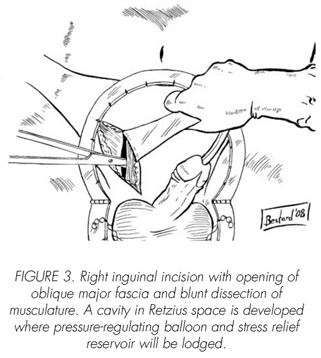
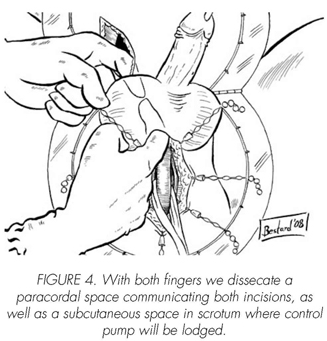
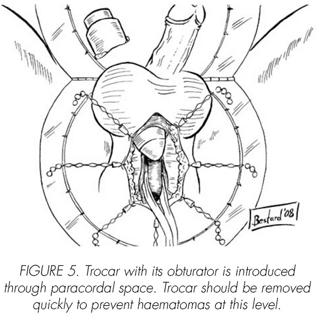
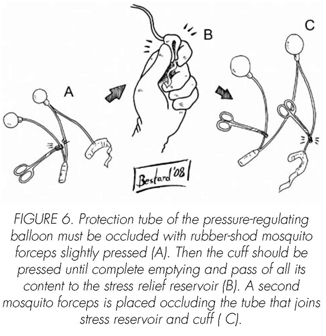
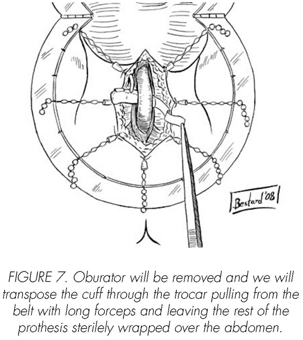
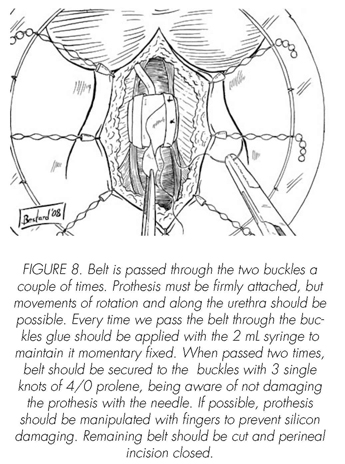
Postoperatory
Urethral catheter may be removed 24 hours after surgery. Prothesis is left deactivated and patient presents null or slight improvement of his incontinence. If continence is referred, he must be encouraged to press the pump to urinate when scrotal edema begins to diminish.
At the first visit 4 weeks after surgery patient will be examined and, if incontinence is still present, we will inject percutaneously 4 to 6 mL of saline serum through its self-sealing port. With this maneuver pressure in the system will be between 40 and 60 cm H2O, which is usually enough to maintain continence at rest with no tissular damage.
If incontinence persists at following visits, new doses of saline serum should be injected, not exceeding 2 mL at each visit (3).
Results
Surgical time was of 90 minutes. Prothesis was submerged in a solution of 200 cc of saline serum with 240 mg of gentamicine for antibiotic prophylaxis. Moreover, 1 gram of amoxicillin/clavulanic acid was administered parenterally before intervention. Postoperatively, 500 mg of Amoxicillin/clavulanic acid was administered orally every 8 hours for 7 days. Patient presented uneventful postoperatory, removing urethral catheter 24 hours after surgery and discharging patient 72 hours after surgery.
Discussion
Since last eighties, diffusion of radical prostatectomy for treatment of localized prostate cancer has increased then number of patients presenting stress incontinence after surgery. Therapeutic options offered to this patients are pelvic floor rehabilitation, pharmacological treatment with duloxetine, intraurethral injections of expansive substances, implantation of suburethral slings and implantation of an artificial sphincter. This last option is the one achieving best results in patients with severe stress incontinence (4). In 1947 Foley reported the first artificial sphincter, consisting on a cuff that achieved continence by inflating around the penis, but provoking erection at the same time (5).
Nowadays, indications for implantation of urethral sphincter are, in decrescent order, post-surgical urinary incontinence (after radical prostatectomy or transurethral resection of the prostate), incontinence due to congenital abnormalities (like spina bifada), incontinence after spinal injuries, neurogenic bladder and, at last, stress incontinence in women when other reconstructive surgical techniques have failed.
Urinary sphincter AMS-800TM has been the only model disposable for many years . It was patented by American Medical Systems in 1983 and, in spite of its excellent results, presented a serie of disadvantages that have been improved in the FlowSecure model (6).
FlowSecure is a single-piece prothesis while AMS-800TM consists of 3 pieces. This theoretically diminish the risk of mechanical failure as there are is no need of connection between its elements (3).
AMS-800TM prothesis presented 3 different reservoirs which transferred different pressure to the cuff. A cuff with pressures between 71-80 cm H2O was recommended when implantation had to be performed on the bladder neck, and a cuff with pressure between 61-70 cm H2O when implantation had to be performed on bulbar urethra. Incontinence resolution was observed only postoperatively and, if it was no resolved, a new intervention was required to implant a higher pressure reservoir. On the other hand, when an insufficient 61-70 cm H2O pressure reservoir had to be changed for a 71-80 cm H2O pressure reservoir, difference of pressure could be between 1 and 19 cm H2O, with no knowledge of which pressure supported the urethra at each moment and which would be the minimal pressure increase necessary for continence (6).
Self-sealing port of FlowSecureTM allows percutaneous manipulation and individual adjustment to each patient, just by injecting or extracting saline serum.
AMS-800TM makes constant pressure over the urethra the same in valsalva as when patient rests. FlowSecureTM increases pressure in the cuff just if intraabdominal pressure increases, transmitting pressure from the stress reservoir immediatly to the cuff. When pressure increase stops cuff turns back to the initial pressure (conditional oclusion system). With this system rest pressure doesn't exceed 40 cm H2O, minimizing damage over the urethra.
FlowSecureTM cuff is also different from cuff of AMS-800TM. This last model presented 3 pillows concentrating pressure over 3 different points of the urethra. Instead, FlowSecureTM presents a circular cuff that adapts to the urethra of each patient and transmits equal pressure all over its surface. This prevents damage over the urethra and cracks on the cuff (3).
AMS-800TM was originally designed to be implanted on the bladder neck, as erosions are less frequent in this zone. But when vascularization of this area is compromised (like happens after prostatic surgery), bulbar urethra is the only possible place (6). FlowSecureTM has been originally designed to be implanted over bulbar urethra with no risk of erosions. Its length of 7 cm makes it suitable to be adapted to most of bladder necks, including women (3).
Conclusions
FlowSecureTM, is a prothesis that adopts advantages from AMS-800TM but offering changes to minimize mechanical failure and reduce harm over the urethra. Its implantation is easy and quick, and as their use is extended we would compare if its theoretical advantages from its antecessor are reflexed in better results and better quality of life for the patient.
 Correspondence:
Correspondence:
Juan E. Bestard Vallejo
Servicio de Urología
Hospital Vall d'Hebron
Passeig Vall d'Hebron 119-129
08035 Barcelona. (Spain)
38625jbv@comb.es
Accepted for publication: Juny, 5th 2008.
References and recomended readings (*of special interest, **of outstanding interest)
*1. Craggs MD, Chaffey NJ, Mundy AR. A preliminary report on a new hydraulic sphincter for controlling urinary incontinence. J Med Eng Technol., 1991; 15:58. [ Links ]
2. García Montes F, Knight SL, Greenwell T, et al. Esfínter Urinario Artificial FlowSecureTM: Un nuevo concepto de esfínter artificial regulable y con oclusión condicional para la incontinencia urinaria de esfuerzo. Actas Urol Esp., 2007; 31:752. [ Links ]
**3. García Montes F, Vicens Vicens A, Ozonas Moragues M et al. Implantación del Esfínter Urinario Artificial FlowSecureTM en uretra bulbar: Descripción de la técnica quirúrgica paso a paso". Actas Urol Esp., 2007; 31:872. [ Links ]
4. Queipo Zaragoza JA, Chicote Pérez F, Borrell Palanca A et al. Tratamiento de la incontinencia urinaria de esfuerzo post-prostatectomía radical mediante malla anclada a ramas isquiopubianas. Actas Urol Esp, 2005; 29:764. [ Links ]
5. Foley FEB. An artificial sphincter: a new device and operation for control of enuresis and urinary incontinence. General considerations, indications and results. J Urol., 1947; 58:250. [ Links ]
*6. García Montes F, Gómez Sancha F, Mundy A. El esfínter urinario artificial. Arch Esp Urol, 2000; 53:201. [ Links ]











 texto en
texto en 


